Figure 5.
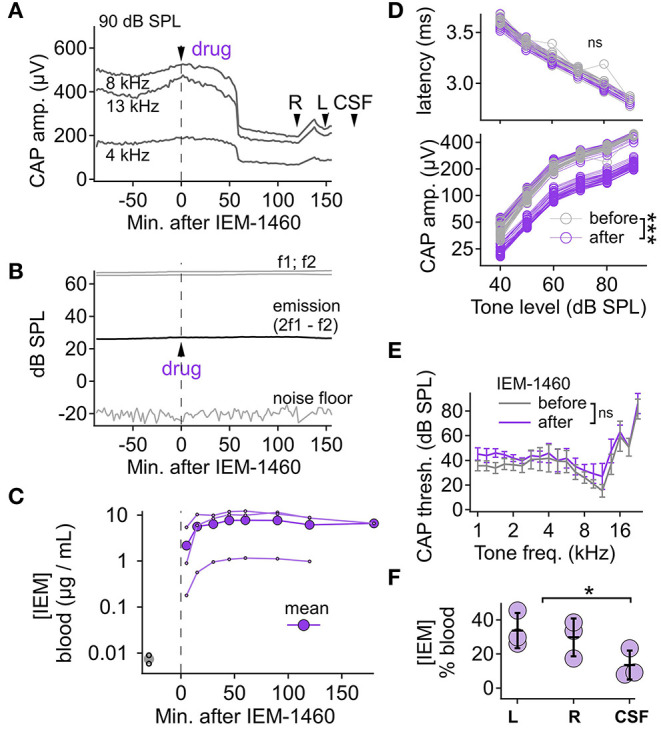
Pharmacokinetic analysis and auditory functional measurements with systemic IEM-1460. (A) After baseline recording, IEM-1460 was injected (IP, 13.5 mg/kg) at time zero. CAP amplitude measured every 2 min for 90 dB SPL tones at 4, 8, and 13 kHz. At the end of the experiment, perilymph samples were taken from right (R) and left (L) cochleae before taking the CSF sample at times indicated. CAP N1-P1 amplitude decreased after drug injection. (B) Distortion product otoacoustic emissions (2f1-f2) remained stable after drug injection. (C) Blood plasma levels of IEM-1460 increased to a sustained maximum within 30 min of injection (N = 3 animals). (D) CAP P1 latency was not significantly different (p = 0.421, Wilcoxon, N = 4) after drug injection (upper) while CAP N1-P1 amplitude was significantly reduced (p < 0.001, Wilcoxon, N = 4) (lower). (E) CAP threshold as a function of cochlear frequency was not significantly different (p > 0.100 across all frequency comparisons, Mann-Whitney U, N = 4) before and after systemic injection of IEM-1460. (F) Perilymph and CSF levels, as % of IEM-1460 concentration in blood, were not significantly different from each other (p = 0.670; Friedman test; N = 3). *p < 0.05; ***p < 0.001.
