Figure 2.
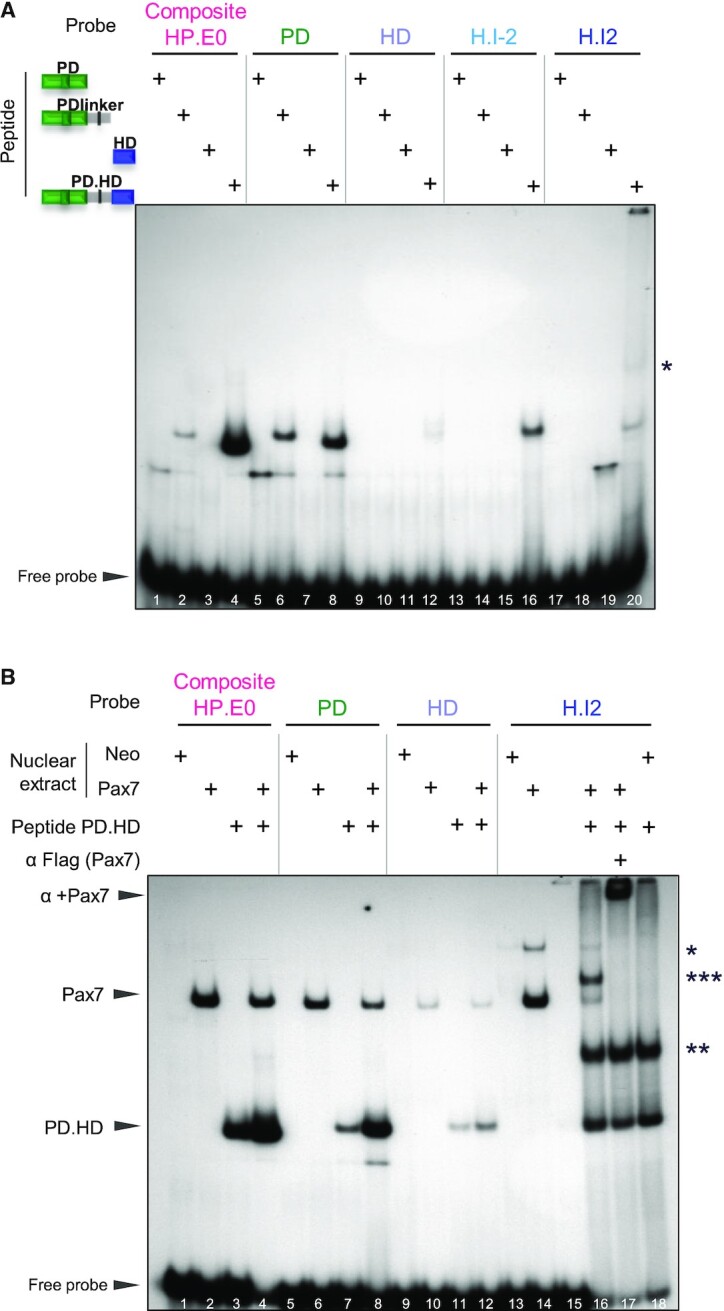
Relative roles of Pax7 PD and HD domains in DNA binding. (A) In vitro binding (EMSA) of various Pax7 polypeptides produced in E. coli to contain the represented portions of the Pax7 DNA binding domains. The purified polypeptides were incubated with 32P-labeled probes before electrophoresis. For each probe, the strongest band likely represents monomer Pax7 polypeptide binding except for the H.I2 probe where a likely dimer band is observed (*). (B) EMSA mixing experiment showing that Pax7 monomers bind the composite site. EMSA was performed for each DNA probe using either AtT20 nuclear extracts expressing (or not, neo) Pax7 with/without the PD.HD polypeptide as indicated. Arrowheads indicate the positions of wild-type Pax7 and PD.HD polypeptide monomer complexes. For the H.I2 probe, dimer bands are also observed for Pax7 (*) and PD.HD (**) together with appearance of a new band (***) in the combined reaction (lane 16) that likely represents a heterodimer between intact Pax7 and PD.HD polypeptide. The presence of Flag-Pax7 in complexes is ascertained by supershifts with the Flag M2 antibody.
