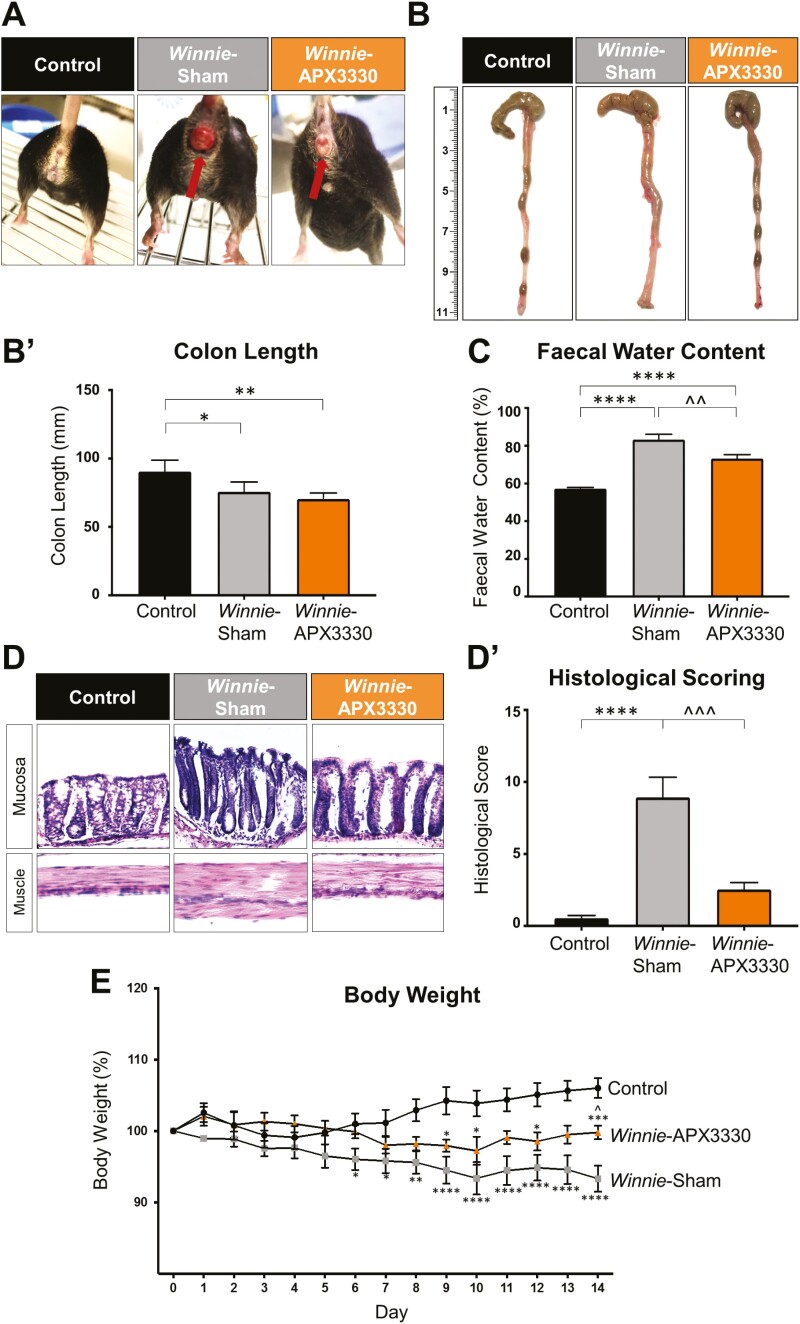
FIGURE 1.
The effects of APX3330 treatment on clinical symptoms in Winnie mice. A, Images of a C57BL/6 control mouse and a Winnie sham-treated mouse with severe intestinal inflammation and rectal prolapse with blood vessel proliferation and edema before and after 14 days of APX3330 treatment. B, B’. Colons from C57BL/6 control, Winnie sham-treated and Winnie APX3330-treated mice were excised and their length (mm) measured at day 15 (n = 5/group). C, Fecal water content (the difference between the wet and dry weight) was calculated as a percent of the wet weight of fresh fecal pellets measured at day 14 of treatment (n = 7/group). D, D’. Gross morphology of the distal colon was assessed by H&E staining and histological scoring was quantified in distal colon cross-sections from C57BL/6 control, Winnie sham-treated and Winnie APX3330-treated mice (n = 5/group). E, Body weight loss or gain in C57BL/6 control (n = 10), Winnie sham-treated (n = 10) and Winnie APX3330-treated (n = 8) mice over the 14-day period. Data expressed as mean ± SEM, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 compared with C57BL/6 control mice; ^P < 0.05, ^^P < 0.01 compared with Winnie sham-treated mice.