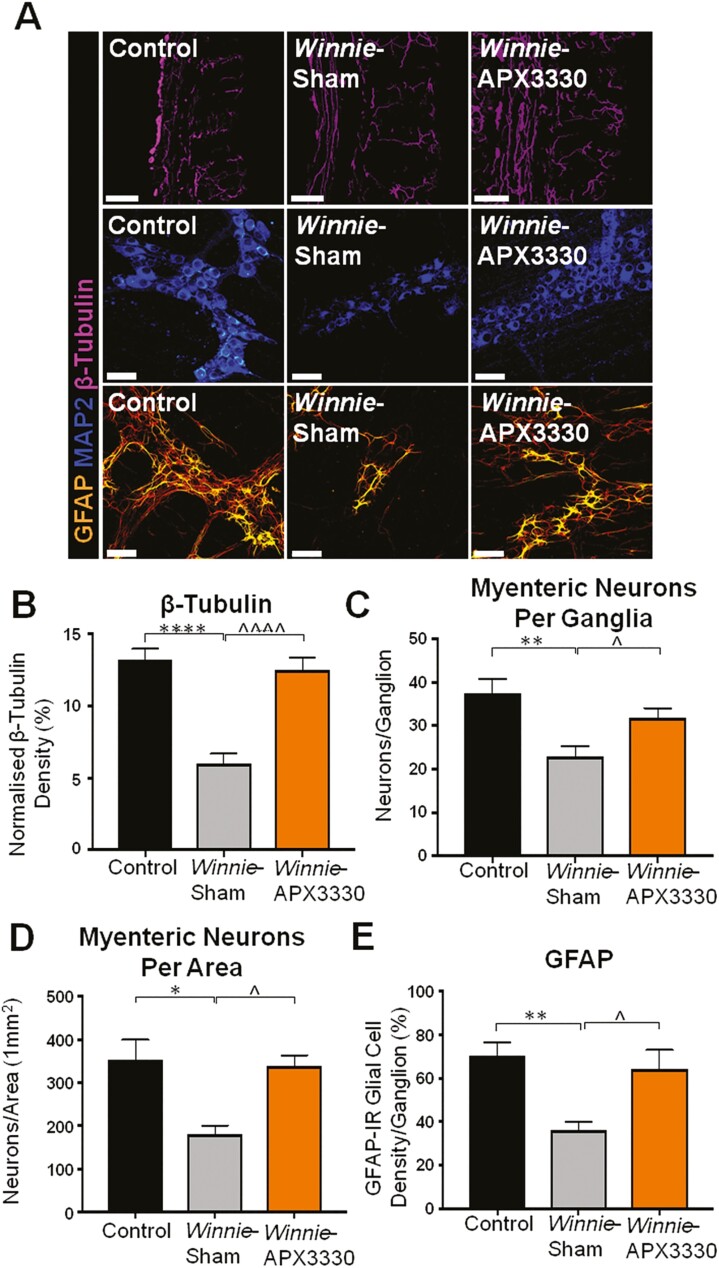
FIGURE 5. THE APX3330 T.
reatment promoted myenteric neuronal survival and ameliorated nerve fiber and glial cell density in the myenteric plexus in Winnie mice. A, Neuronal microtubule proteins were labeled by immunofluorescence using β-tubulin (III; purple) antibody to identify nerve fibers innervating the colon in cross-sections (scale bar = 100 µm, 20x magnification). Anti-MAP2-IR antibody staining (blue) for myenteric neurons in LMMP preparations (scale bar = 50 µm, x40 magnification). Glial cells were labeled with an anti-GFAP antibody (orange) in the myenteric plexus of the colon from C57BL/6 control, Winnie sham-treated, and Winnie APX3330-treated mice (scale bar = 50 µm, 40x magnification). B, Density of β-tubulin (III)-IR nerve fibers normalized to colon thickness in C57BL/6 control, Winnie sham-treated, and Winnie APX3330-treated mice (n = 5/group). C, Quantitative analysis of myenteric neurons per ganglion C57BL/6 control, Winnie sham-treated, and Winnie APX3330-treated mice (n = 5/group). D, Myenteric neurons were quantified per area in LMMP preparations of C57BL/6 control, Winnie sham-treated, and Winnie APX3330-treated mice (n = 5/group). E, Density of GFAP-IR glial cells per ganglia in C57BL/6 control, Winnie sham-treated, and Winnie APX3330-treated mice (n = 5/group). Data expressed as mean ± SEM, *P < 0.05, **P < 0.01, ***P < 0.001, compared with C57BL/6 control mice; ^P < 0.05, ^^P < 0.01, ^^^P < 0.001 compared with Winnie sham-treated mice.