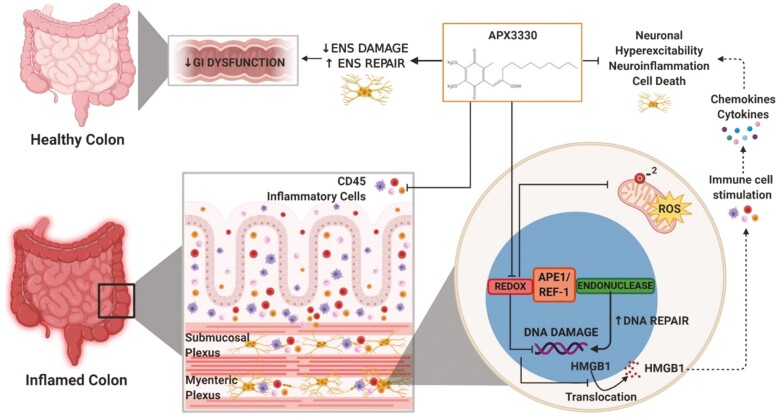
FIGURE 9.
Effects of APX3330 treatment on enteric neurons and GI functions. In the inflamed colon, high levels of colonic leukocyte infiltration and fecal Lcn-2 were alleviated by APX3330 treatment. Chronic intestinal inflammation associates with the damage to the enteric nervous system embedded within the intestinal wall and controls gastrointestinal (GI) functions. In enteric neurons, APX3330 treatment APX3330 reduces oxidative stress via inhibition of APE1/Ref-1’s redox signaling, mitochondrial superoxide production, and oxidative DNA damage. However, suppressing APE1/Ref-1 redox activity enhances APE1/Ref-1’s endonuclease repair activity. DNA damage induces translocation of HMGB1 from the nucleus to the cytoplasm. Acetylated cytosolic HMGB1 released from cells into extracellular space interacts with immune cells and stimulates production of cytokines and chemokines leading to neuroinflammation, hyperexcitability, and neuronal death (dashed line: not studied in our model). After APX3330 treatment, HMGB1 was retained in the nuclei of myenteric neurons. Targeting this pathway with APX3330 ameliorated enteric neuropathy, ameliorated colonic dysmotility, and altered GI transit leading to improved clinical signs associated with chronic colitis (created with BioRender.com).