Figure 5.
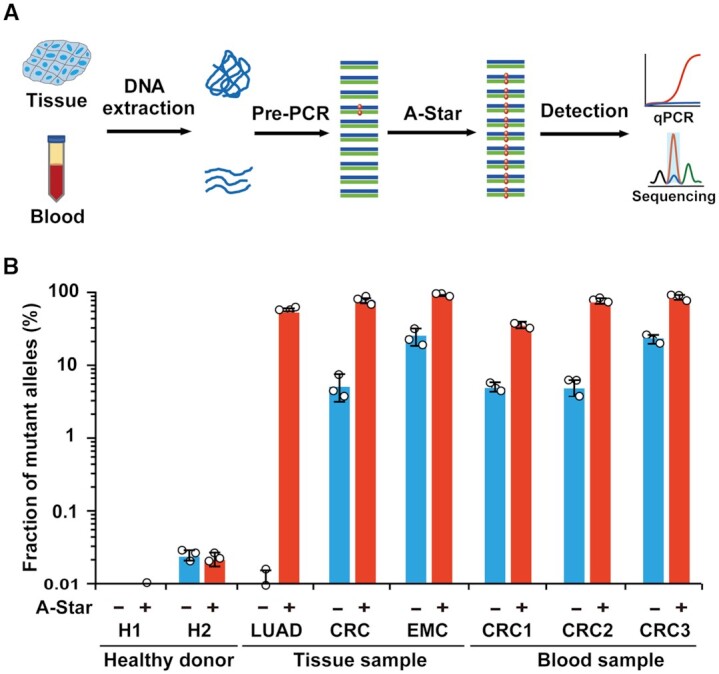
Analysis of A-Star with human clinical samples. (A) Flow chart of the A-Star procedure for the identification of cancer-associated SNVs in human samples. (B) Evaluation of A-Star by TaqMan qRT-PCR using clinical samples from diverse cancer types, as exemplified by KRAS G12D-containing samples from patients with lung adenocarcinoma (LUAD), colorectal cancer (CRC), or endometrial cancer (EMC). The control reactions did not contain gDNA pairs. Error bars represent standard deviations of the mean, n = 3.
