Abstract
Objective
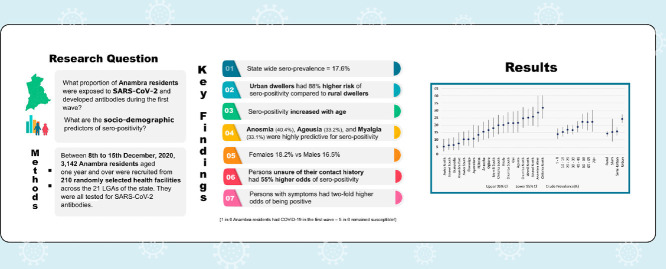
The objective of this study was to determine the proportion of the Anambra State population that had been infected by the SARS-CoV-2 virus and developed antibodies before the second wave.
Methods
The WHO-recommended health facility-based cross-sectional approach was adapted for this survey. Between 8th and 15th December 2020, 3142 participants across the 21 local government areas (LGAs) of the State, aged one year and over, attending randomly selected health facilities, were recruited. Demographic and symptom-related information were collected from the participants as well as whole peripheral blood, which was tested for SARS-CoV-2 IgG and IgM with rapid test kits.
Results
425 participants tested positive for IgG only, 74 for IgM only, while 54 were positive for both IgG and IgM. Overall, 553 positives were recorded, giving a crude seroprevalence of 17.6% (95% CI = 16.26 – 18.98). It ranged widely from 31.9% (95% CI = 24.43 – 40.22) in Onitsha North LGA to 5.4% (95% CI = 2.19 – 10.78) in Awka north. Bayesian Adjustments yielded a state seroprevalence of 16.1%.
Conclusion
One in six state residents had been infected by SARS-CoV-2 and developed antibodies before the second wave. All LGAs, age groups, sexes, and settlement types were affected by COVID-19. A large proportion of the population remained susceptible to SARS-CoV-2.
Keywords: SARS-CoV-2 seroepidemiology, COVID-19 immunity, Disease surveillance, Population survey
Graphical Abstract
Introduction
The World Health Organization (WHO) declared the SARS-COV-2 virus infection a public health emergency of international concern (PHEIC) on 30th January 2020 (Eurosurveillance Editorial Team, 2020). About a month later, on 27th February 2020, Nigeria diagnosed her first case of coronavirus disease 2019 (COVID-19) in an international immigrant (Nigeria Centre for Disease Control, 2020a). This first case of COVID-19 triggered a massive nationwide response led by the Nigerian Centre for Disease Control (NCDC) to restrict the disease to the point source as close as possible. Despite the implementation of national widespread control measures, including restriction of movement across states, Anambra State in South-east, Nigeria diagnosed her first case of COVID-19 on 10th April 2020 (Nigeria Centre for Disease Control, 2020b). The pandemic response at the State level was led by the State's Ministry of Health (MoH) Public health emergency operations center (PHEOC) under the technical oversight and guidance of the NCDC and the Directorate of public health and disease control in the State MoH. The State's initial control efforts included intense contact investigations, enforcement of physical distancing, hand and respiratory hygiene at the population level, risk communication, isolation of suspected cases, and case management. However, following the diagnosis of more epidemiologically unlinked cases in the weeks to months that followed, established community transmission of SARS-Cov-2 was declared in certain Local Government Areas (LGAs) of the State, including Awka South Onitsha South (Anambra state ministry of health, 2020a).
Nigeria, in mid-December 2020, announced the existence of a COVID-19 second wave in the country (Nigeria Centre for Disease Control, 2020c). Before the second wave in Nigeria, there was a relaxation of lockdown rules imposed by the Federal and State Governments. As a result, Nigerians reverted to their everyday lives with minimal effort to maintain the public health measures of hand and respiratory hygiene, face masks in public, and maintaining physical distancing. Despite this behavior, the increase in the number of new infections was not pronounced, leading to the closure of isolation and treatment centers in Anambra State and several other States. Given these observations, the widespread occurrence of sub-clinical infection amongst many Anambra residents could not be ruled out, nor had it been established. This knowledge gap remains true for many parts of the country. Thus, in line with the WHO's proposal, the Anambra State Ministry of Health conducted a population seroprevalence survey for SARS-CoV-2 antibodies among Anambra State residents. As of 15th November 2020, the State had tested a total of 7,552 samples from suspected COVID-19 cases and diagnosed 285 confirmed cases therefrom, giving a positivity rate of 3.8% (Anambra state ministry of health, 2020b). Healthcare worker infections accounted for 11.9% (34/285) of all cases in the State. A total of 19 deaths were recorded, giving an overall case fatality rate of 6.7%, more than triple the national CFR, which was 1.8% (1,166/66,228) as of 21st November (Anambra state ministry of health, 2020b; Nigeria Centre for Disease Control, 2020d).
At the time of commencement of the study, all 21 Local government Areas (LGAs) in the State had reported at least a confirmed COVID-19 case with 74.0% of the cases (211/285) confined to five LGAs: Awka South, Idemili North, Onitsha North, Onitsha South, and Nnewi North (Anambra state ministry of health, 2020c). There was a male to female ratio of 60:40 amongst the confirmed cases. Most cases were spread between the ages of 11–50 years, with the age group 11– 40 years accounting for 61% (173/285) of the cases. The positivity trend, which tilted towards lower ages, could be due to the massive testing of school children - Fifty-two cases were diagnosed amongst children and young people aged between 10-20 years (18.2% of positive cases) (Anambra state ministry of health, 2020c).
The true extent of exposure to the SARS-CoV-2 virus to sub-populations within the State is crucial for public health action, but remains unknown. Thus, a key objective of this study was to determine the proportion of the Anambra State population that had been infected by the SARS-CoV-2 virus and generated an antibody response. Secondarily was to investigate for socio-demographic factors associated with the exposure.
Methods
Setting
This survey was conducted in Anambra State in Southeast Nigeria. The State is surrounded by five States, namely Delta State to the west, Imo and Rivers States to the south, Enugu State to the east, and Kogi State to the north. The major urban areas in Anambra State are Awka, Onitsha, and Nnewi. The indigenous ethnic groups are Igbo (98% of the population) and a small population of Igala (2% of the population) that live in the north-western part of the State (www.anambrastate.gov.ng/history/, 2020). It has a landmass area of 4,844 km2, with coordinates between Latitude 6° 20′N and Longitude 7° 00′E. Anambra has tropical rain forest vegetation, a humid climate with an average temperature of about 30°C, and rainfall between 152cm – 203cm Brief History of Anambra State 2021.
Administratively, the State is divided into 21 Local Government Areas (LGA). The sampling and recruitment of participants for this survey were designed along the lines of these LGAs. Also, there are 330 wards, and 177 communities in the state, with the capital territory at Awka.
Anambra Bureau of Statistics provided the 2020 projected population of Anambra State to be 6,149,744; of which males are 3,124,070 (50.8%) and females 3,025,674 (49.2%) and a population growth rate of 2.21% per annum (Anambra state ministry of health, 2017). On the whole, about 70% of the State's population is below the age of 30 across the sexes. The sampling for this study and analysis of the findings took into account this population age structure.
Study design
This study is a population-based, age-stratified, one-time seroprevalence survey of all Anambra residents one year of age and above. This design is one of the three standard methods recommended by the WHO for SARS-CoV-2 population prevalence surveys (World Health Organization, 2020a). The logistical impediments to the iterative or prospective approaches made this design the preferred option for our study.
Sampling
The survey was based in health facilities (World Health Organization, 2020a) and adopted a multi-stage sampling approach for ease of administration. In the first stage of the sampling process, all local government areas (LGA) in the State were selected for the survey. For each LGA, a serialized list of all public and private health facilities with a monthly client load of at least 100 was obtained from the State Ministry of Health's Directorate for planning research and statistics. The health facilities’ list formed the sampling frame for the second stage of the sampling process at the LGA level, from which five to thirteen facilities were selected by simple random sampling depending on the projected population of the LGA. In the third stage, fifteen eligible consenting participants were recruited consecutively from each selected health facility. Out of this quota, two participants were to be healthcare staff whose duties involve routine close interactions with patients.
For the sample size calculation, each LGA was considered to have a sufficient population. Given that the true population prevalence of the SARS-CoV-2 antibodies was unknown in our setting, we assumed an anticipated prevalence of 50%. Alpha level was set at 5%, and a power of 80% was desired. We inflated the initial estimate of the minimum sample size by a design effect of 1.5 to account for clustering along LGAs. The WHO-recommended open-source software, OpenEpi.com (World Health Organization, 2020a; Sullivan, Dean, Soe, 2009), was utilized for the sample size calculation. Considering the impact of COVID-19-related infodemic (misinformation) in the State and apprehension towards testing, we accounted for a possible rejection rate of up to 20%.
Using the State's population age structure, we computed the proportional sample fraction for each age group to ensure adequate age representation.
Recruitment
All clients (≥ one year of age) and health staff were eligible for the survey from each selected health facility. Clients and staff that declined consent were excluded from the study. Consenting participants were serially recruited until the recruitment quota of 15 participants was reached. Trained healthcare workers used an adapted version of the WHO-recommended questionnaire (World Health Organization, 2020a) to collect participant's relevant clinical and socio-demographic information (appendix 1). Where necessary, the information was explained in the predominant local language to enhance understanding. Data were collected on 21 symptoms of COVID-19 developed from symptoms listed in the WHO recommended survey tool and the state PHEOC symptom checklist. Also, each participant's pinprick blood sample was used for the SARS-CoV-2 antibodies Serological test. The survey started on 8th December 2020 and was concluded by 15th December in all health facilities.
Two drops of whole blood from the finger prick of each participant's preferred hand were used for SARS-CoV-2 IgG and IgM antibodies testing with the Realy Tech SARS-CoV-2 Antibodies Rapid Test Device. The rapid test procedure followed the manufacturer's guidelines (Technical File for Realy Tech Rapid Test Device 2020) and the result read within 10 – 15 minutes and recorded in the corresponding datasheet on the questionnaire. Each participant was assigned a two-digit code number written on the questionnaire and the test kit's ID space. After testing and recording the result, a photograph of the test kit (showing the result section and participant's code) was taken and sent to the supervising team.
Data analysis
The Realy Check immunoassay kit used for the survey tested for the presence of SARS-CoV-2 IgG and IgM antibodies. A positive result could be for either or both. Although we presented findings for each antibody, the crude and adjusted prevalence calculations were based on a dichotomized positive (IgG + IgM) or a negative outcome. Simple frequencies were calculated as well as other descriptive statistics relevant to the sample. Chi-squared hypothesis tests were conducted to test the association between exposure factors and categorical outcomes, and the significance value was set at 5%. Univariable and multivariable logistic regression analyses (using the simultaneous inclusion of variables) were carried out to clarify relationships between exposures and seropositivity. Crude and adjusted Odds ratios are presented with associated 95% confidence intervals, estimated using the exact binomial method. Exposure variables that showed significant associations were included in the final multiple regression model. Analyses were carried out in IBM SPSS version 25. Using crude prevalence measures, we plot high-low-close charts, spatial dot maps (Appendix 3), and sex-disaggregated population graphs of the positivity rates (Appendix 3). Further details of the data analysis procedures are included in Appendix 3.
Bayesian adjustment of prevalence
The SARS-CoV-2 IgG and IgM rapid test kits utilized for the study were produced by Hangzhou Realy Tech Ltd and had reported sensitivity and specificity values determined in a pretest of 200 patients (Technical File for Realy Tech Rapid Test Device 2020). For SARS-CoV-2 specific IgG, a pre-determined sensitivity and specificity of 99% and 100%, respectively, were reported. Similarly, for IgM, sensitivity and specificity were given as 98% and 99%, respectively. We chose the lower test sensitivity of 98% and specificity of 99% for IgM for our adjustment. We adjusted our crude prevalence measure to account for the rate of false positives that may have occurred due to the test kit used. Using the Bayesian conditional probability equation, we estimated the probability of actually having SARS-CoV-2 antibody given a positive test from the kits (Greenland, Robins, 1991). Details in Appendix 3.
The findings imply a 91.3% probability of having the SARS-CoV-2 antibody, given that one tested positive using our test kits. We applied this probability to all our prevalence measures across the State and LGAs.
Role of the funding source
This study was funded by the Government of Anambra state through the COVID-19 intervention fund under the State Ministry of Health. The funding source had no role in determining the design of this study or the data collection and analyses plan. They played no role in the writing of this report as well as the decision to submit the manuscript for publication. All the authors had full access to the data from this study and bear the responsibility for submitting this manuscript for publication.
Results
On the whole, data from 3142 participants tested at the 210 participating health facilities across the 21 LGAs of the State were included in the analyses as summarized in Table 1 . Although an initial 3150 persons were recruited at the sites, six declined while two entries lacked critical information on demographics and symptom screening and hence were excluded from the analyses. 37.2% (1168) of the participants were males. The median age for males was 28 years (IQR = 13 – 47), whilst that of females was 33 (IQR = 20 – 45). The 70+ age group had 134 participants and contributed the least of all the age groups to the sample (4.3%), while the 20 – 29 age group with 648 (20.6%) contributed the highest. The number of participants surveyed at each LGA ranged from 74 in Ayamelum (Population of 226,826) to 238 in Idemili North (population of 616,835). In our sample, only 28 persons (0.9%) definitely had contact with an RT-PCR diagnosed case of COVID-19. 486 (15.5%) reported not being sure of the status of persons they had come in contact with, while the majority of participants, 2628 (83.6%), did not contact a COVID-19 case. In terms of places of abode, 46% (1449) of the participants reside in rural areas whilst the urban, Semi-Urban, and Slum areas had 29% (918), 21% (677), and 2.4% (77), respectively. Healthcare workers numbered 419, making up 13.3% of the sample; 1140 (36.2%) reported experiencing none of the 21 symptoms of SARS-CoV-2 infection listed in the survey tool, which was adapted from the WHO recommended survey questionnaire.
Table 1.
Characteristics of Survey participants
| Population | Sex | Age | Contact with COVID-19 case | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | Sample | Female (%) | 1 – 9 (%) | 10 – 19 (%) | 20 – 29 (%) | 30 – 39 (%) | 40 – 49 (%) | 50 – 59 (%) | 60 – 69 (%) | 70 + (%) | No (%) | Yes (%) | Unknown (%) | |
| Overall | 5,982,242 | 3142 | 1974 (62.8) | 388 (12.3) | 523 (16.6) | 648 (20.6) | 527 (16.8) | 455 (14.5) | 268 (8.5) | 199 (6.3) | 134 (4.3) | 2628 (83.6) | 28 (0.9) | 486 (15.5) |
| Aguata | 530,047 | 133 | 84 (63.2) | 11 (8.3) | 12 (9.0) | 33 (24.8) | 46 (34.6) | 16 (12.0) | 10 (7.5) | 5 (3.8) | 0 (0) | 114 (85.7) | 1 (0.8) | 18 (13.5) |
| Anambra East | 213,574 | 92 | 59 (64.1) | 14 (15.2) | 18 (19.6) | 17 (18.5) | 11 (12.0) | 11 (12.0) | 8 (8.7) | 10 (10.9) | 3 (3.3) | 81 (88.0) | 0 (0) | 11 (12.0) |
| Anambra West | 239,722 | 77 | 46 (59.7) | 10 (13.0) | 14 (18.2) | 13 (16.9) | 13 (16.9) | 11 (14.3) | 7 (9.1) | 5 (6.5) | 4 (5.2) | 50 (64.9) | 0 (0) | 27 (35.1) |
| Anaocha | 408,093 | 154 | 108 (70.1) | 17 (11.0) | 30 (19.5) | 34 (22.1) | 22 (14.3) | 26 (16.9) | 7 (4.5) | 13 (8.4) | 5 (3.2) | 116 (75.3) | 2 (1.3) | 36 (23.4) |
| Awka North | 161,242 | 130 | 81 (62.3) | 15 (11.5) | 18 (13.8) | 26 (20.0) | 20 (15.4) | 25 (19.2) | 14 (10.8) | 5 (3.8) | 7 (5.4) | 84 (64.6) | 3 (2.3) | 43 (33.1) |
| Awka South | 270,698 | 183 | 116 (63.4) | 25 (13.7) | 29 (15.8) | 39 (21.3) | 32 (17.5) | 24 (13.1) | 14 (7.7) | 15 (8.2) | 5 (2.7) | 162 (88.5) | 2 (1.1) | 19 (10.4) |
| Ayamelum | 226,826 | 74 | 47 (63.5) | 11 (14.9) | 10 (13.5) | 16 (21.6) | 11 (14.9) | 9 (12.2) | 10 (13.5) | 5 (6.8) | 2 (2.7) | 64 (86.5) | 1 (1.4) | 9 (12.2) |
| Dunukofia | 138,008 | 151 | 108 (71.5) | 13 (8.6) | 16 (10.6) | 29 (19.2) | 29 (19.2) | 34 (22.5) | 18 (11.9) | 9 (6.0) | 3 (2.0) | 131 (86.8) | 0 (0) | 20 (13.2) |
| Ekwusigo | 226,570 | 144 | 79 (54.9) | 16 (11.1) | 25 (17.4) | 33 (22.9) | 20 (13.9) | 20 (13.9) | 14 (9.7) | 8 (5.6) | 8 (5.6) | 101 (70.1) | 1 (0.7) | 42 (29.2) |
| Idemili North | 616,835 | 238 | 159 (66.8) | 32 (16.4) | 42 (17.6) | 47 (19.7) | 37 (15.5) | 40 (16.8) | 18 (7.8) | 12 (5.0) | 10 (4.2) | 190 (79.8) | 6 (2.5) | 42 (17.6) |
| Idemili South | 297,380 | 150 | 96 (64.0) | 21 (14.0) | 22 (14.7) | 36 (24.0) | 20 (13.3) | 19 (12.7) | 15 (10.0) | 10 (6.7) | 7 (4.7) | 144 (96.0) | 2 (1.3) | 4 (2.7) |
| Ihiala | 432,658 | 162 | 117 (63.9) | 27 (14.8) | 33 (18.0) | 35 (19.1) | 26 (14.2) | 21 (11.5) | 16 (8.7) | 13 (7.1) | 12 (6.6) | 168 (91.8) | 1 (0.5) | 14 (7.7) |
| Njikoka | 212,586 | 162 | 101 (62.3) | 20 (12.3) | 28 (17.3) | 25 (15.4) | 31 (19.1) | 21 (13.0) | 16 (9.9) | 10 (6.2) | 11 (6.8) | 135 (83.3) | 3 (1.9) | 24 (14.8) |
| Nnewi North | 225,622 | 198 | 122 (61.6) | 25 (12.6) | 40 (20.2) | 41 (20.7) | 31 (15.7) | 24 (12.1) | 14 (7.1) | 14 (7.1) | 9 (4.5) | 173 (87.4) | 0 (0) | 24 (12.6) |
| Nnewi South | 334,573 | 172 | 114 (66.3) | 21 (12.2) | 31 (18.0) | 36 (20.9) | 24 (14.0) | 22 (12.8) | 16 (9.3) | 12 (7.0) | 10 (5.8) | 158 (91.9) | 0 (0) | 14 (8.1) |
| Ogbaru | 317,707 | 150 | 93 (62.0) | 20 (13.3) | 29 (19.3) | 32 (21.3) | 26 (17.3) | 18 (12.0) | 12 (8.0) | 9 (6.0) | 4 (2.7) | 136 (90.7) | 0 (0) | 14 (9.3) |
| Onitsha North | 178,904 | 144 | 87 (60.4) | 18 (12.5) | 26 (18.1) | 29 (20.1) | 20 (13.9) | 21 (14.6) | 8 (5.6) | 13 (9.0) | 9 (6.3) | 102 (70.8) | 3 (2.1) | 39 (27.1) |
| Onitsha South | 195,685 | 156 | 85 (54.5) | 15 (9.6) | 31 (19.9) | 33 (21.2) | 27 (17.3) | 26 (16.7) | 13 (8.3) | 6 (3.8) | 5 (3.2) | 114 (73.1) | 0 (0) | 42 (26.9) |
| Orumba North | 246,865 | 161 | 93 (57.8) | 16 (9.9) | 28 (17.4) | 40 (24.8) | 28 (17.4) | 24 (14.9) | 9 (5.6) | 9 (5.6) | 7 (4.3) | 147 (91.3) | 0 (0) | 14 (8.7) |
| Orumba South | 268,048 | 154 | 96 (62.3) | 19 (12.3) | 18 (11.7) | 32 (20.8) | 29 (18.8) | 24 (15.6) | 15 (9.7) | 9 (5.8) | 8 (5.2) | 138 (89.6) | 1 (0.6) | 15 (9.7) |
| Oyi | 240,599 | 136 | 83 (61.0) | 22 (16.2) | 23 (16.9) | 22 (16.2) | 24 (17.6) | 19 (14.0) | 14 (10.3) | 7 (5.1) | 5 (3.7) | 120 (88.2) | 2 (1.5) | 14 (10.3) |
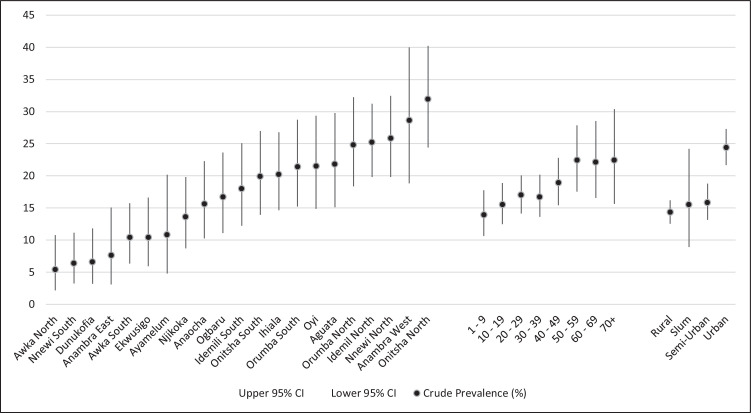
The SARS-CoV-2 antibody rapid tests carried out for all participants yielded 553 positives giving a crude seroprevalence of 17.6% (95% CI = 16.26 – 18.98); 425 (76.9%) tested positive for IgG only, 74 (13.4%) were positive for IgM only, while 54 (9.8%) tested positive for both IgG and IgM. There were six invalid test results representing 0.2% of the results. Across the LGAs, the crude prevalence ranges from 31.9 (95% CI 24.43 – 40.22) in Onitsha North to 5.4% (95% CI 2.19 – 10.78) in Awka North. Figure 1 is a high-low-close chart that summarizes the crude prevalence and 95% CI of all LGAs, age groups, and residence types in the State.
Figure 1.
A high-low-close chart displaying the crude prevalence and associated 95% confidence intervals in Anambra State
The female crude prevalence was 18.2% (95% CI 16.56 – 20.01) and was not significantly higher than that of males at 16.5% (95% CI = 14.44 – 18.78). Residents of Urban areas had a crude prevalence of 24.4% (95% CI = 21.65 – 27.31) which was significantly higher than those of the residents of Rural, Semi-urban and Slum areas with 14.3% (95% CI = 12.52 – 16.19); 15.8% (95% CI = 13.14 – 18.77) and 15.5% (95% CI = 8.92 – 24.22), respectively. There was no significant difference in the crude prevalence between healthcare workers (17.2% [95% CI = 13.7 – 21.14]) and non-healthcare workers (17.7% [95% CI = 16.29 – 19.2). Comparatively, the older age groups had a higher prevalence than the younger age groups with age ‘1 – 9’ having the lowest (13.9% [95% CI = 10.63 – 17.77]) while ‘70+’ had the highest (22.4% [95% CI 15.64 – 30.39]). The Chi-square for trend analysis showed that contact history was associated with a significant difference in crude prevalence (table B appendix 3). Participants who definitely contacted a known COVID-19 case had a crude prevalence of 28.6% (95% CI = 13.22 – 48.67) higher than those who did not contact a known case. Of note, participants with an unknown social contact history had a crude prevalence of 25.1% (95% CI = 21.31 – 29.21). Participants who reported not having any of the symptoms had about half the crude prevalence of those with a symptom(s).
Crude prevalence measures were adjusted for the reported sensitivity and specificity to account for the false positives from the Realy Check immunoassay test kits. The adjustments were made using the Bayesian conditional probability theorem, as described earlier. Adjusted prevalence measures (displayed in table A of appendix 3) indicate that the SARS-CoV-2 antibody prevalence in the state was 16.1%.
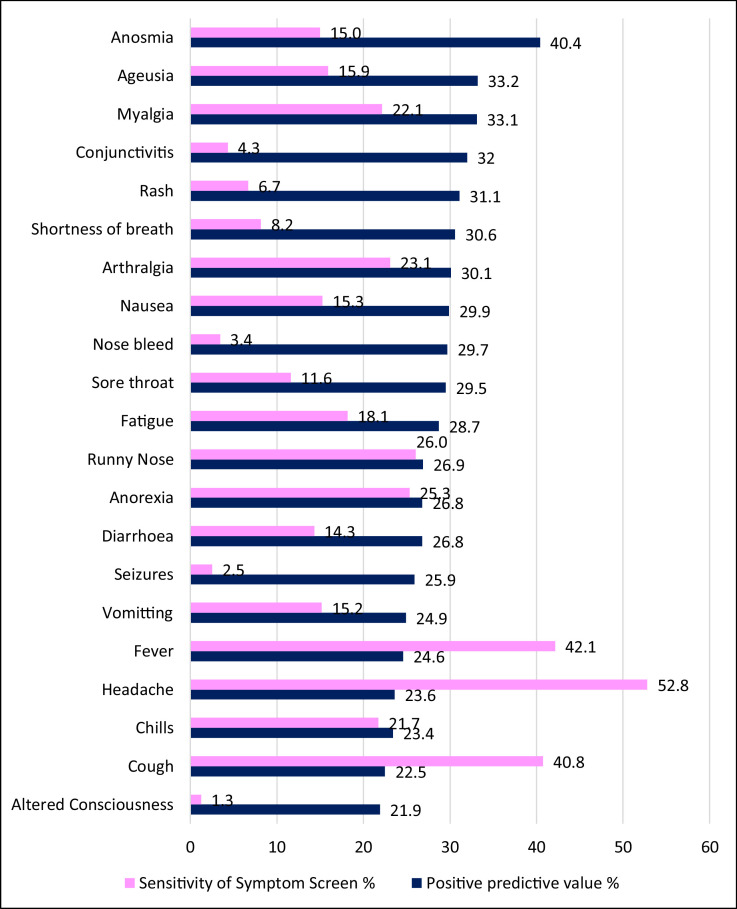
The symptoms data summarized in Figure 2 showed that Anosmia, Ageusia, and Myalgia with 40.4%, 33.2%, and 33.1% had the highest positive predictive values for SARS-CoV-2 seropositivity. However, the most commonly occurring symptoms were headache, fever, and cough, having been experienced by 52.8%, 42.1%, and 40.8% of symptomatic participants, respectively.
Figure 2.
Positive predictive value and prevalence of SARS-CoV-2 symptoms in Anambra State
To quantify the increased risk of seropositivity across the various sub-categories of the participants, Table 2 displays the crude and adjusted odds ratios (aOR) with associated 95% confidence intervals. Urban dwellers had more than 88% (aOR = 1.88 [95% CI = 1.52 – 2.34]) greater odds of testing positive compared to rural dwellers. Although all age groups from 40 and above tended to have higher odds of testing positive, ranging from 50% higher odds for the '40 – 49′ group to 82% greater risk in the ‘70+’ group, the confidence intervals overlapped. Notably, persons with unknown contact history exhibited 55% higher adjusted odds of testing positive for the SARS-CoV-2 antibodies with aOR of 1.55 (95% CI = 1.22 – 1.96). Persons who reported symptoms had almost two-fold odds of testing positive for the antibodies (aOR = 1.91 [95% CI = 1.54 – 2.37]). The model was adjusted for age, residence type, contact, and symptom history.
Table 2.
Socio-demographic Factors and Associated Crude/Adjusted Odds Ratios of SARS-CoV-2 Seropositivity
| SARS-CoV-2 Antibody Positive | ||||||||
|---|---|---|---|---|---|---|---|---|
| N | OR | 95% CI | p-value | aOR | 95% CI | p-value | ||
| Type of Residence | Rural | 1450 | Ref | |||||
| Urban | 918 | 1.94 | 1.57 – 2.39 | 0.0001 | 1.89 | 1.52 –2.34 | 0.0001 | |
| Semi-Urban | 677 | 1.13 | 0.88 – 1.45 | 0.36 | 1.13 | 0.87 – 1.46 | 0.35 | |
| Slum | 97 | 1.1 | 0.62 –1.94 | 0.75 | 1.23 | 0.07 –2.19 | 0.48 | |
| Age band | 1 – 9 | 388 | Ref | |||||
| 10 –19 | 523 | 1.13 | 0.78– 1.65 | 0.51 | 1.15 | 0.79 –1.67 | 0.48 | |
| 20 – 29 | 648 | 1.27 | 0.89 – 1.80 | 0.19 | 1.33 | 0.93 – 1.91 | 0.12 | |
| 30 – 39 | 527 | 1.24 | 0.86 – 1.79 | 0.25 | 1.36 | 0.93 –1.97 | 0.11 | |
| 40 – 49 | 455 | 1.44 | 0.10 – 2.09 | 0.05 | 1.50 | 1.03 – 2.19 | 0.03 | |
| 50 – 59 | 268 | 1.78 | 1.19 – 2.68 | 0.01 | 1.89 | 1.24 – 2.85 | 0.00 | |
| 60 – 69 | 199 | 1.76 | 1.13 – 2.73 | 0.01 | 1.87 | 1.19 – 2.93 | 0.01 | |
| 70 + | 134 | 1.78 | 1.09 –2.94 | 0.02 | 1.82 | 1.10– 3.03 | 0.02 | |
| Contact history | No Contact | 2628 | Ref | |||||
| Contact | 28 | 2.09 | 0.91– 4.77 | 0.08 | 1.57 | 0.67 –3.65 | 0.29 | |
| Unknown | 486 | 1.75 | 1.39 –2.20 | 0.0001 | 1.55 | 1.22 – 1.96 | 0.0001 | |
| Symptom status | Asymptomatic | 1140 | Ref | |||||
| Symptomatic | 2002 | 2.03 | 1.65 – 2.51 | 0.0001 | 1.91 | 1.54 – 2.37 | 0.0001 | |
Ref: Reference category for the multivariable logistic regression model.
Discussion
The result of this large seroprevalence survey using health facility attendees from all local government areas (LGAs) in Anambra State, confirms the magnitude of the community spread of the infection before the second wave of COVID-19. It is clear from the results that the epidemic had affected all age groups, sexes, settlement types, and LGAs.
The survey found an overall SARS-CoV-2 seroprevalence of approximately 16%, which suggests that before the second wave of COVID-19 in Nigeria in mid-December 2020, about 1 in 6 of all Anambra state residents were already infected with SARS-CoV-2. This prevalence is similar to the recent NCDC household survey results from Lagos, Enugu, and Nasarawa States of Nigeria, as contained in an NCDC press release (Abubakar et al., 2021). Despite this high infection rate found by the survey, less than one percent of all participants reported prior contact with known COVID-19 patients, thus confirming the community spread of the disease in the State. Unsurprisingly, Onitsha North LGA, which plays host to the largest market in West Africa and is known for the high mobility of its population, boasts the highest prevalence. Findings amongst a similar mobile population of Kenyan truck drivers and assistants showed a high adjusted seroprevalence of 42.3% (95% CI = 38.4 – 46.3) (Kagucia et al., 2021).
It has been established that the SARS-CoV-2 IgG antibody appears about 2 – 3 days after the development of the IgM in infected humans but, while the IgM antibodies disappear after a few weeks, the IgG antibodies persist for months or years (World Health Organization, 2020b). This work showed that about 80% of the participants were seropositive for IgG only, suggesting that they were infected with the SARS-CoV-2 infection months preceding the survey. Also, less than 10% of the participants were seropositive for IgM only, which should disappear after a few weeks of infection, suggesting a declining incidence of new SARS-CoV-2 infections in Anambra State before the second wave. This assumption is consistent with the COVID-19 situation in the State at the onset of the survey, including the closure of isolation/treatment centers.
The survey has further confirmed a higher urban prevalence of SARS-CoV-2 infections when compared to rural areas and other residential area sub-categories in the State. An earlier Ivorian survey found a significant association between city-dwelling and seropositivity amongst mineworkers from three different sites (Milleliri et al., 2021).
Somewhat surprisingly, the seroprevalence among health workers did not differ from that of non-health workers. In similar settings, a crude seroprevalence of 45.1% was found amongst asymptomatic healthcare workers in Ibadan southwest Nigeria (Olayanju et al., 2020), 13 – 36% from Congo Kinshasa (Ndaye et al., 2021), and 20.8% from three different counties in Kenya (despite inter-county differences)(Etyang et al., 2021) Our finding could be a reflection of an early policy priority of the state to protect healthcare workers through provision of PPEs, strict enforcements of prevention measures, and training on infection prevention and control. It is also entirely plausible that some 'non-healthcare workers could have been at higher risk; these include petrol pump attendants, law enforcement agents, and public transport operators.
It was expected that participants that had social contacts with known COVID-19 patients would have significantly higher SARS-CoV-2 seroprevalence when compared with those without social contacts. On the contrary, the survey showed that the participants with unknown social contact history were significantly more likely to be SARS-CoV-2 seropositive when compared with those that reported social contacts with known COVID-19 patients. Furthermore, Anambra residents older than 39 seemed to have significantly higher odds of being infected with SARS-CoV-2 compared to the 10 – 39 years age groups. This contrasts the child and adolescent predominance of the reported cases in the State since the large number of school children tested (using RT-PCR) prior to the resumption of schools skewed the age distribution of reported cases (Anambra state ministry of health, 2020c).
Of note, the loss of sense of smell (anosmia) or taste (ageusia) predicted the SARS-CoV-2 infection among participants more than other symptoms. This is similar to earlier findings from other settings (Lan et al., 2020). Therefore, it would be safer to rule out COVID-19 when any State resident presents with such symptoms. Furthermore, the survey confirms asymptomatic COVID-19 in Anambra State; about 24% of seropositive participants were without any of the COVID-19 symptoms defined by the WHO for the disease. Although the recall bias inherent with this study design is acknowledged, the true prevalence to reported cases ratio will likely be large (Murhekar, Clapan, 2021).
In conclusion, the SARS-CoV-2 seroprevalence of 16.07% among residents of Anambra State confirms widespread community transmission of the disease in the State before the onset of the second wave in December 2020. The SARS-CoV-2 infection rate varied across age groups and was highest among residents above 49 years. Also, seroprevalence did not differ between males and females nor between health workers and non-health workers. The disease prevalence rate was higher among urban dwellers and amongst those who were uncertain of the COVID-19 disease status of their social contacts. In our environment, the clinical picture of COVID-19 shared key symptoms with some commonly occurring diseases such as Malaria and Tuberculosis, but a loss of smell and loss of taste were most predictive of seropositivity. A large proportion of the population remains highly susceptible to SARS-CoV-2 infection. There is a need to strengthen the enforcement of COVID-19 prevention messages and strategies to minimize the community spread of the disease.
Limitations
We acknowledge some limitations to this study. Resource limitations restricted us to using a rapid immunoassay test instead of an ELISA test or a combination of the two tests; however, it has been shown that the two tests have comparable accuracy for SARS-CoV-2 antibodies (Traugott et al., 2020). We also relied on the manufacturer's reported sensitivity and specificity determined in a non-African population because our local validation of the test kits during the second wave of the COVID-19 showed similar findings (described in Appendix 3). Furthermore, given that information on exposure and outcomes were obtained simultaneously, there was a possibility of information bias on account of recall, especially as regards variables such as symptoms and contact history but, it was probably nondirectional and would not have substantially affected the study outcomes. Despite these drawbacks, the findings will help local public health efforts such as risk communication and resource allocation and clinical symptom-based screening in our settings with limited access to RT-PCR.
Acknowledgments
Data sharing
The de-identified dataset from this survey can be made available upon reasonable request through a letter addressed to the Honorable Commissioner for Health, the Anambra State Ministry of Health. The letter should detail the intended use of the data, which will be verified by the department of research planning and statistics of the state ministry of health. Data will be released for purposes of research, including meta-analyses. Requests should be sent to the first author's email: vincentokpala@gmail.com, or by post to the Anambra State Ministry of Health, Jerome Udoji Secretariat, Aroma Awka, Anambra, Nigeria.
Acknowledgments
This study was funded by the Anambra State Government through their COVID-19 intervention fund. We acknowledge the 210 health facility focal persons that conducted this survey in the selected health facilities, 21 LGA supervisors, 21 LGA focal persons, and the two data entry officers. We especially thank the participants for consenting to be part of this vital research. The authors acknowledge the technical support from the World Health Organization field office in Awka.
Funding source Declaration of interests
OgoChukwu Vincent Okpala, Simeon Onyemaechi, Obiageli Uchebo, Ugochukwu Chukwulobelu, Chuma Emembolu, Ben Okoye, Uchenna Benedict Okoye, Nelly Chibuzor Dike, Anastasia Obiageli Odumegwu, Christopher Ideh, Vincent Chinedu Okpala, Peter Ikenna Okoye, Maryann Chinyere Enike, and Oluchi Onyedikachi are employed by the Anambra state government as salaried staff of the State ministry of health.
Cyril Chukwudi Dim, Chukwuebuka Immanuel Ugwu, Chukwumuanya Igboekwu, and Christiana Okoye have no interests to declare.
This study was funded by the Anambra State Government through their COVID-19 intervention fund.
Ethical considerations
In Nigeria, this intervention falls within the definition of Public health surveillance (in emergency response); nevertheless, ethical clearance was obtained from the Directorate of planning research and statistics of the Anambra state ministry of health. Before recruitment, each participant was counseled, and informed verbal consent was obtained. The counseling explained in clear terms the purpose of the survey, the voluntariness of participation, the potential risks, benefits to the individual and their community, as well as our commitment to maintaining confidentiality. To ensure anonymity, the LGAs, HFs, and participants were coded as appropriate – only these codes were written on participants' questionnaire/datasheet. The consent form is attached as Appendix 2.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ijid.2021.07.040.
Appendix. Supplementary materials
References
- Abubakar I., Dalglish S.L., Ihekweazu C.A., Bolu O., Aliyu S.H. Lessons from co-production of evidence and policy in Nigeria's COVID-19 response. BMJ global health. 2021;6(3) doi: 10.1136/bmjgh-2020-004793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anambra state ministry of health, 2017. Anambra state strategic health development plan ii (2017–2021)
- Anambra state ministry of health, 2020a. Public health emergency operations center COVID-19 Situation Report 13th July 2020.
- Anambra state ministry of health, 2020b. Public health emergency operations center COVID-19 Situation Report 16th November 2020.
- Anambra state ministry of health, 2020c. Public health emergency operations center COVID-19 Situation Report 13th December 2020.
- Brief History of Anambra State. Available at: https://anambrastate.gov.ng/history/ (Accessed:29th November 2020) 2021.
- Etyang A.O., Lucinde R., Karanja H., Kalu C., Mugo D., Nyagwange J., Gitonga J., Tuju J., Wanjiku P., Karani A., Mutua S. Seroprevalence of Antibodies to SARS-CoV-2 among Health Care Workers in Kenya. medRxiv. 2021 [Google Scholar]
- Eurosurveillance Editorial Team Note from the editors: World Health Organization declares novel coronavirus (2019-nCoV) sixth public health emergency of international concern. Eurosurveillance. 2020;25(5) doi: 10.2807/1560-7917.ES.2020.25.5.200131e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenland S., Robins J.M. Empirical-Bayes adjustments for multiple comparisons are sometimes useful. Epidemiology. 1991:244–251. doi: 10.1097/00001648-199107000-00002. [DOI] [PubMed] [Google Scholar]
- Kagucia E.W., Gitonga J.N., Kalu C., Ochomo E., Ochieng B., Kuya N., Karani A., Nyagwange J., Karia B., Mugo D., Karanja H.K. Seroprevalence of anti-SARS-CoV-2 IgG antibodies among truck drivers and assistants in Kenya. medRxiv. 2021 [Google Scholar]
- Lan F.Y., Filler R., Mathew S., Buley J., Iliaki E., Bruno-Murtha L.A., Osgood R., Christophi C.A., Fernandez-Montero A., Kales S.N. COVID-19 symptoms predictive of healthcare workers' SARS-CoV-2 PCR results. PloS one. 2020;15(6) doi: 10.1371/journal.pone.0235460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milleliri J.M., Coulibaly D., Nyobe B., Rey J.L., Lamontagne F., Hocqueloux L., Giaché S., Valery A., Prazuck T. SARS-CoV-2 infection in Ivory Coast: a serosurveillance survey among goldmine workers. The American Journal of Tropical Medicine and Hygiene. 2021;104(5):1709. doi: 10.4269/ajtmh.21-0081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murhekar M.V., Clapham H. COVID-19 serosurveys for public health decision making. The Lancet Global Health. 2021;9(5):e559–e560. doi: 10.1016/S2214-109X(21)00057-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigeria Centre for Disease Control,2020a. COVID-19 Outbreak in Nigeria Situation Report. S/N 001.Available at: https://covid19.ncdc.gov.ng/ (Accessed: 29th November 2020).
- Nigeria Centre for Disease Control,2020b. COVID-19 Outbreak in Nigeria Situation Report. S/N 043. Available at:https://covid19.ncdc.gov.ng (Accessed: 29th November 2020).
- Nigeria Centre for Disease Control 2020c.COVID-19 Situation Report weekly epidemiological report 6 Epi Week47:16th–22ndNovember,2020. Available at: https://covid19.ncdc.gov.ng/(Accessed:29th November 2020).
- Nigeria Centre for Disease Control 2020d. COVID-19 Situation Report weekly epidemiological report 6 Epi Week51:14th–20thDecember,2020. Available at: https://covid19.ncdc.gov.ng (Accessed: 22nd March 2020).
- Ndaye A.N., Hoxha A., Madinga J., Mariën J., Peeters M., Leendertz F.H., Mundeke S.A., Ariën K.K., Tanfumu J.J.M., Kingebeni P.M., Vanlerberghe V. Challenges in interpreting SARS-CoV-2 serological results in African countries. The Lancet Global Health. 2021;9(5):e588–e589. doi: 10.1016/S2214-109X(21)00060-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olayanju O., Bamidele O., Edem F., Eseile B., Amoo A., Nwaokenye J., Udeh C., Oluwole G., Odok G., Awah N. SARS-CoV-2 Seropositivity in Asymptomatic Frontline Health Workers in Ibadan, Nigeria. The American journal of tropical medicine and hygiene. 2020;104(1):91–94. doi: 10.4269/ajtmh.20-1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan K.M., Dean A., Soe MM. On academics: OpenEpi: a web-based epidemiologic and statistical calculator for public health. Public health reports. 2009;124(3):471–474. doi: 10.1177/003335490912400320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Technical File for Realy Tech Rapid Test Device June 2020. Available at: https://pei.ie/wpcontent/uploads/2020/06/Realy_Tech_Techincal_File_PEI_compressed.pdf. (Accessed: 29th November 2020).
- Traugott M, Aberle SW, Aberle JH, Griebler H, Karolyi M, Pawelka E, Puchhammer-Stöckl E, Zoufaly A, Weseslindtner L. Performance of Severe Acute Respiratory Syndrome Coronavirus 2 Antibody Assays in Different Stages of Infection: Comparison of Commercial Enzyme-Linked Immunosorbent Assays and Rapid Tests. J Infect Dis. 2020 Jul 6;222(3):362–366. doi: 10.1093/infdis/jiaa305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . World Health Organization; 2020. Population-based age-stratified seroepidemiological investigation protocol for coronavirus 2019 (COVID-19) infection, 26 May 2020 (No. WHO/2019-nCoV/Seroepidemiology/2020.2) [Google Scholar]
- World Health Organization. What we know about the COVID-19 immune response: THE LATEST ON COVID-19 IMMUNITY & THE CURRENT GLOBAL SITUATION. CORONAVIRUS UPDATE 34. LAST UPDATE: 02 August 2020a https://www.who.int/docs/default-source/coronaviruse/risk-comms-updates/update-34-immunity-2nd.pdf?sfvrsn=8a488cb6_2. (Accessed: 27th February 2020)
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.