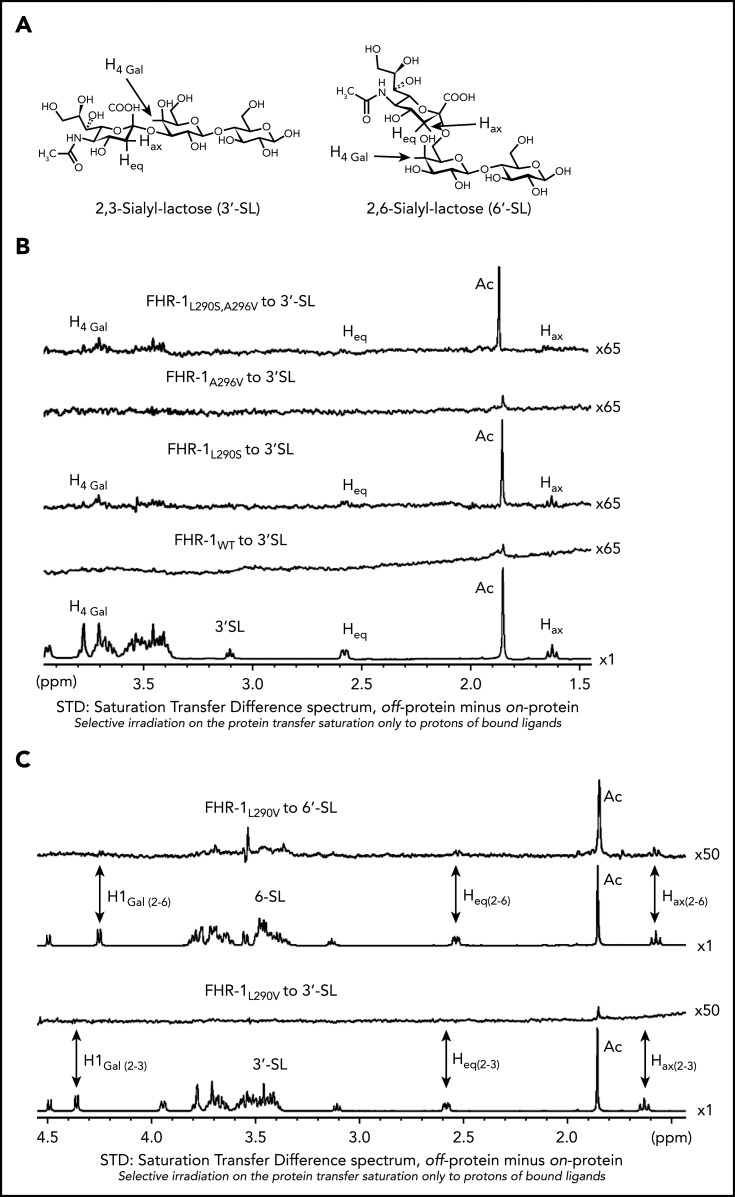
Figure 5.
NMR data for the interaction of FHR-1 proteins with 3′-SL and 6′-SL. (A) Structures of 3′-SL (α-Neu5Ac-[2-3]-β-D-Gal-[1-4]-D-Glc) and 6′-SL (α-Neu5Ac-[2-6]-β-D-Gal-[1-4]-D-Glc). (B) STD-NMR spectra with 3′-SL. Lower panel shows reference spectra of 3′-SL corresponding to the off-resonance spectra acquired in presence of FHR-1WT. Above this are panels showing the STD spectra in presence of FHR-1WT at 9 µM and FHR-1L290S, A296V at 16 µM; the acetate of the 5-NAc group of the sialic residue received the highest saturation. In both cases, 3′-SL concentration was adjusted to 50:1 ligand/protein ratio. (C) STD-NMR spectra of FHR-1L290V mutant with 3′-SL and 6′-SL. From bottom to top, panels show reference spectra of 3′-SL corresponding to the off-resonance spectra in presence of FHR-1L290V, STD-NMR spectra of 3′-SL in presence of FHR-1L290V at 15 µM, reference spectra of 6′-SL corresponding to the off-resonance spectra in presence of FHR-1L290V, and STD-NMR spectra of 6′-SL in presence of FHR-1L290V at 16 µM; the acetate of the 5-NAc group of the sialic residue received the highest saturation. In both cases, 3′-SL and 6′-SL concentrations were adjusted to 50:1 ligand/protein ratio. All STD-NMR spectra were acquired with 3-s saturation on −0.14 ppm. Background signal of protein was subtracted in each case from a spectrum of the protein acquired under the same conditions but in absence of ligand. Signals of selected protons are labeled.