Fig. 1. Study design.
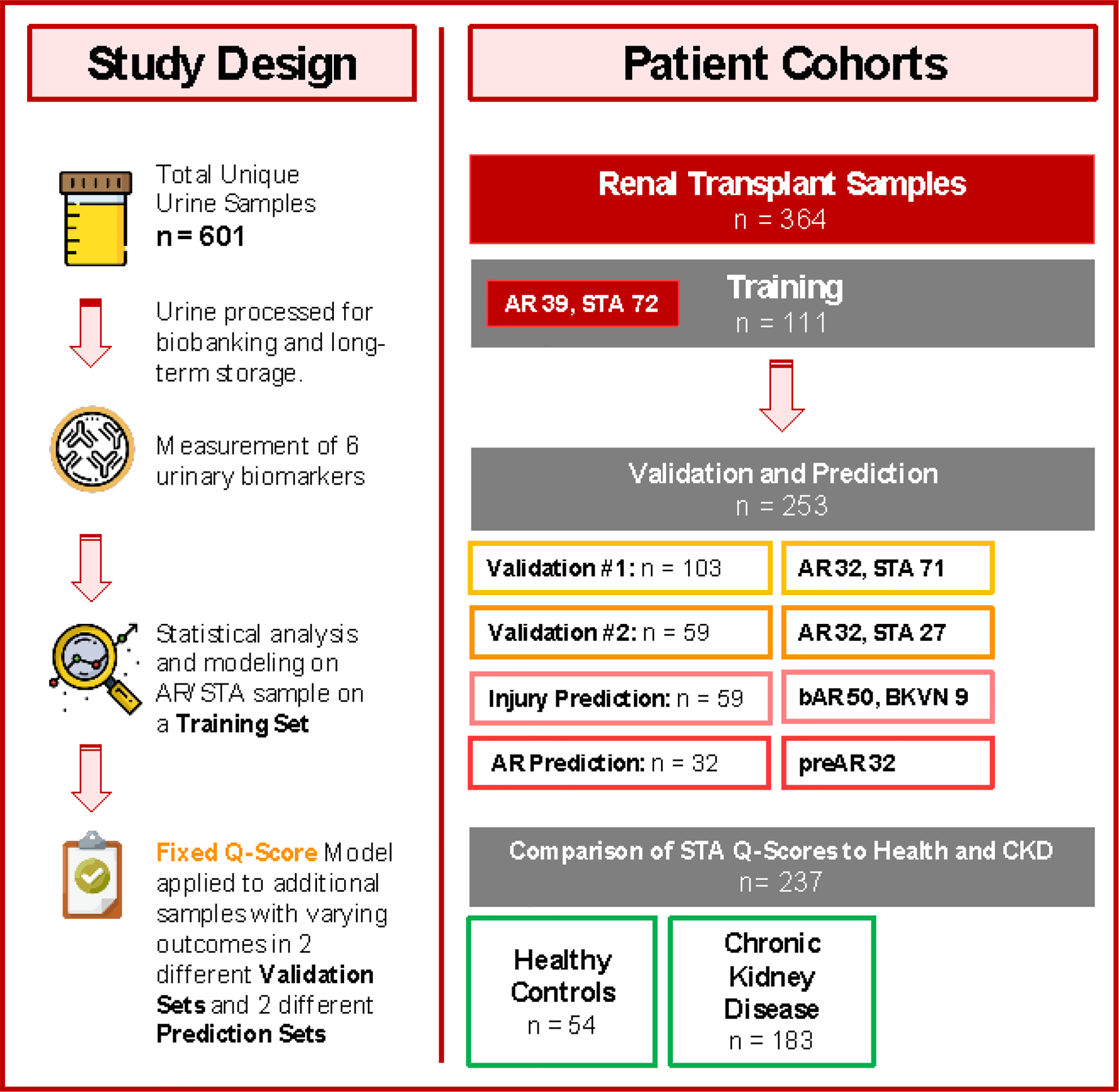
In this multicenter, prospective study, a total of 601 patient samples were assessed. 364 urine samples belonged to renal transplant recipients, where every urine sample was paired with a renal transplant biopsy for phenotype classification into the following diagnoses: stable (STA; n = 170), acute rejection (AR; n = 103), borderline AR (bAR; n = 50), and BK virus nephropathy (BKVN; n = 9). An additional 32 patients with AR had urine samples collected before the rejection episode; these samples were also paired with biopsies. In the context of a stable kidney transplant, we compared patients’ urine scores with a scores run on urine samples from healthy controls without any renal insufficiency (n = 54). In addition, we compared the urine scores from stable kidney transplant patients against the scores from patients with early CKD (stages 1 and 2; n = 183). The selected urinary biomarkers were measured on all urine samples collected, and statistical analysis and modeling were performed on a subset of patients, defined as STA or AR, and split out randomly as a training set (n = 111) for modeling the urine score. The fixed Q-Score model from the training set was then applied to two separate validation sets consisting of STA and AR samples with set sizes of 103 and 59 for validation set 1 and validation set 2, respectively. The fixed urine score was next applied to samples with other transplant injuries (bAR/BKVN; n = 59) and for prediction of rejection in the cohort of patients (preAR samples; n=32) with urine samples collected within 8 months of a biopsy-confirmed AR episode. The preAR samples were collected at a time where the paired biopsy was histologically stable and there was no graft dysfunction.
