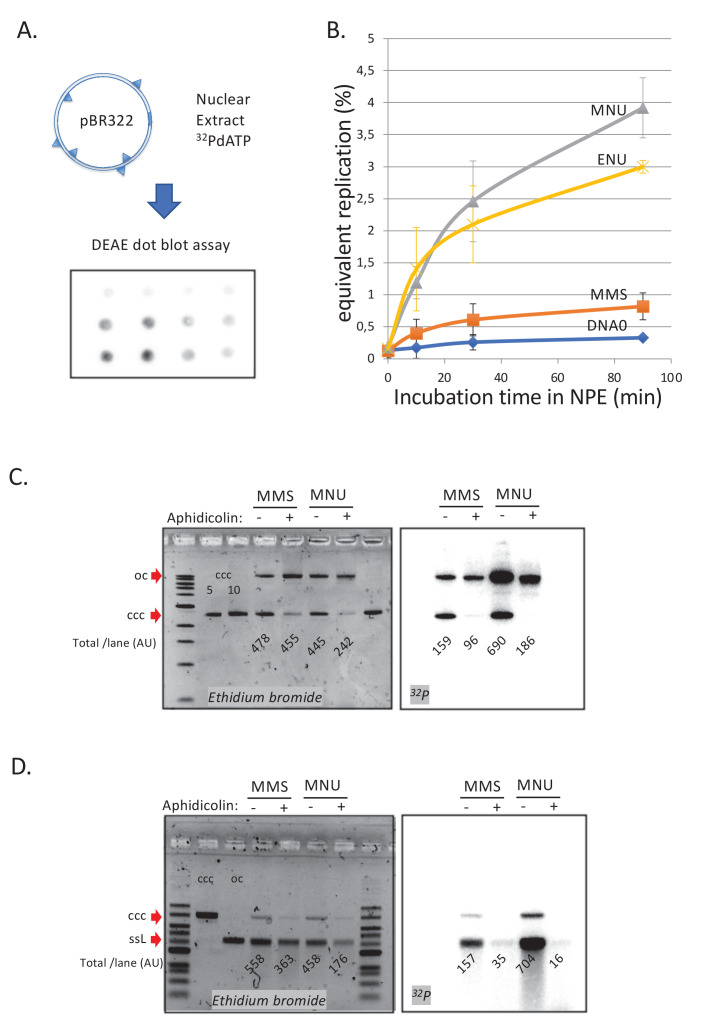
Figure 2. DNA repair synthesis in alkylated and undamaged control plasmid DNA in NPE.
(A) Outline of the spot assay. Plasmids were incubated in nuclear extracts supplemented with α32P-dATP; at various time points, an aliquot of the reaction mixture was spotted on DEAE paper (see 'Materials and methods'). The dot blot is shown for the sake of illustration only. (B) Plasmid DNA pBR322 (4.3 kb) samples, modified to a similar extent with -MMS, -MNU and -ENU, were incubated in nucleoplasmic extracts (NPE) supplemented with α32P-dATP at room temperature; incorporation of radioactivity was monitored as a function of time using the spot assay described above (A). Undamaged plasmid DNA0 was used as a control. At each time point, the average values and standard deviation from three independent experiments were plotted. The y-axis represents DNA repair synthesis expressed as a fraction of input plasmid replication (i.e., 10% means that the observed extent of repair synthesis is equivalent to 10% of input plasmid replication). This value was determined knowing the average concentration of dATP in the extract (50 μM) and the amount of added α32P-dATP. (C) N-methyl-N-nitrosourea (MMS)- and N-methyl-N-nitrosourea (MNU)-treated plasmids were incubated in NPE, supplemented or not, by aphidicolin (150 μM final). After 1 hr of incubation, plasmids were purified and analyzed by agarose gel electrophoresis under neutral loading conditions. The gel was imaged by fluorescence (left: ethidium bromide image) and by autoradiography (right: 32P image). The number below each lane indicates the total amount of signals per lane (expressed in arbitrary units [AU]). Aphidicolin treatment decreases incorporation into MNU-treated plasmid close to fourfold, while it affected incorporation into MMS-treated plasmid only 1.6-fold. (D) Samples as in (C). Gel loading is performed under alkaline conditions to denature DNA before entering the neutral agarose gel, allowing single-stranded nicks present in DNA to be revealed. The number below each lane indicates the amount of signals per lane (AU).