Figure 1.
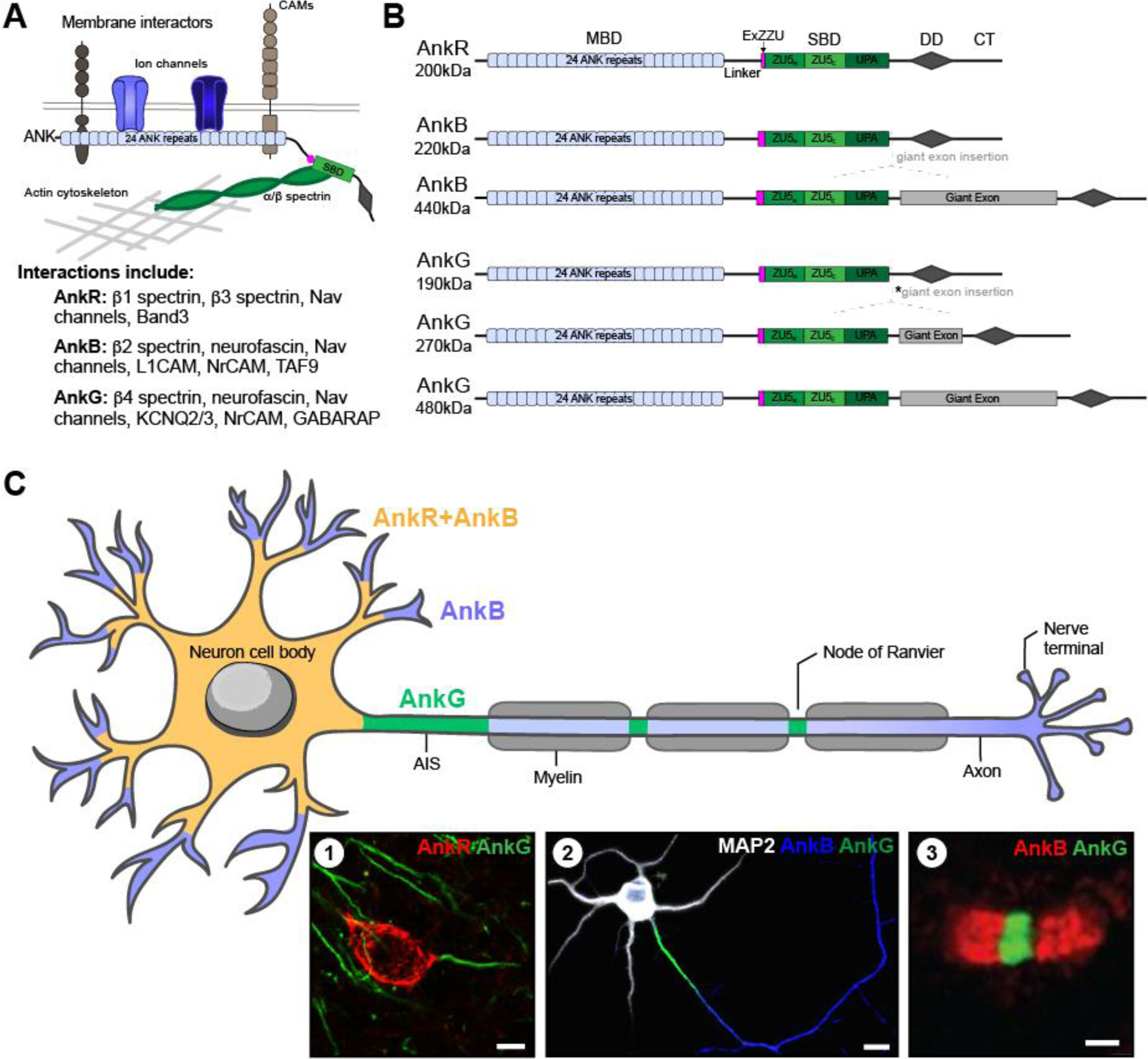
(A) Schematic of subcellular ankyrins with domains and interactions; and list of key interactors for each ankyrin. (B) Primary ankyrin isoforms. AnkR has one main isoform, 200kDa. However, alternative splicing of AnkB results in two main isoforms: 220kDa, and 440kDa. Similarly, alternative splicing of AnkG results in three main isoforms: 190kDa, 270kDa, and 480kDa. The 270kDa isoform results from an in-frame splicing of the giant exon eliminating 1,900 amino acids (*asterisk). Apart from the giant exon present in some isoforms, all three ankyrins have a similar structure. A N-terminal membrane binding domain (MBD) consisting of 24 ANK repeats, a linker domain, a recently discovered extension of the spectrin binding domain (SBD) termed ExZZU thought to enhance ankyrin-spectrin binding affinities, the SBD also known as the ZZU domain due to its three parts (ZU5N, ZU5C, and UPA), the DEATH domain (DD), and a regulatory C-terminal tail. (C) Schematic of ankyrin localization in neurons. All three ankyrins are expressed in neurons; however, each one has a unique pattern of localization. AnkB (blue) is expressed throughout neurons, however in some, AnkR is co-localized with AnkB at the soma and proximal dendrites (yellow). Whereas AnkG (green) is found at the AIS and nodes of Ranvier. Insets show (1) immunolabeling of AnkR and AnkG in adult mouse cortical neurons; scale bar, 10µm; (2) MAP2 (white) labeling the somatodendritic domain, AnkB (blue) co-localized with MAP2 in the somatodendritic domain but is also present in the distal axon, and AnkG (green) labeling the AIS in vitro rat hippocampal neurons at DIV10 [modified from 12]; scale bar, 10µm; and (3) Schwann cell derived paranodal AnkB (red), and AnkG (green) at a node of Ranvier in mouse sciatic nerve [modified from 11]; scale bar, 1µm.
