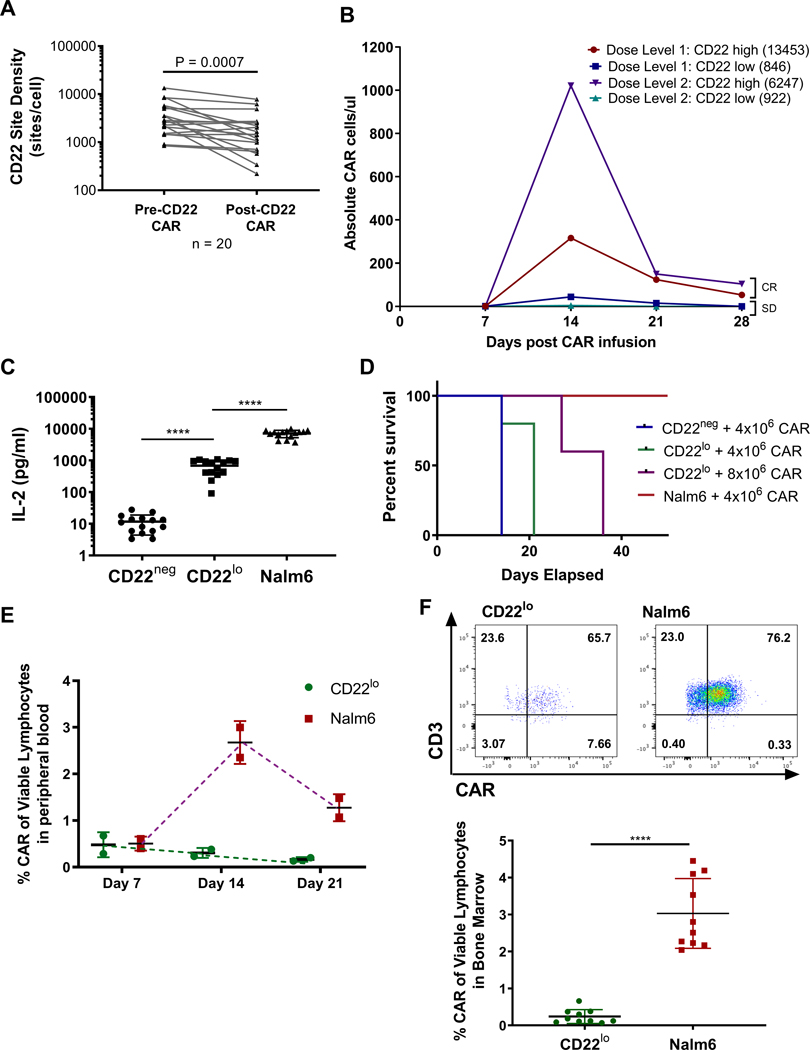
Figure 1: CD22 expression decreases following CD22 CART, and decreased CD22 expression attenuates CART expansion and persistence.
(A) Patient samples pre- and post-CD22 CAR therapy were evaluated for CD22 expression using Quantibrite-PE bead evaluation. Statistics were performed using Wilcox test. (B) Four separate CD22 CART patient samples – two at Dose level 1 and two at Dose level 2 – were evaluated for site density using Quantibrite-PE analysis and CD22 CAR using flow cytometry. (CR – MRD-negative Complete Response; SD – Stable Disease) (C) 1×105 tumor cells were co-cultured with 1×105 CD22 CAR cells from CD22 CART patient samples for 18hrs. Supernatant was evaluated by Meso Scale Multiplex pro-inflammatory cytokine panel. Statistics were calculated using unpaired t-test (**** p<0.0001, *** p<0.0002, ** p<0.0021). (D) Kaplan-Meier curves comparing CD22neg to CD22lo and Nalm6 leukemia-bearing mice with different CART doses. (E) Peripheral blood was collected from mice at interval timepoints and assessed for CAR expansion using flow cytometry and analyzed on Fortessa flow machine. (F) CAR expansion was evaluated from bone marrow of mice 16 days after tumor injection. Cells were stained for flow cytometry and analyzed on Fortessa flow machine. Statistics were calculated using Mann-Whitney test (**** p < 0.0001, * p < 0.0332).