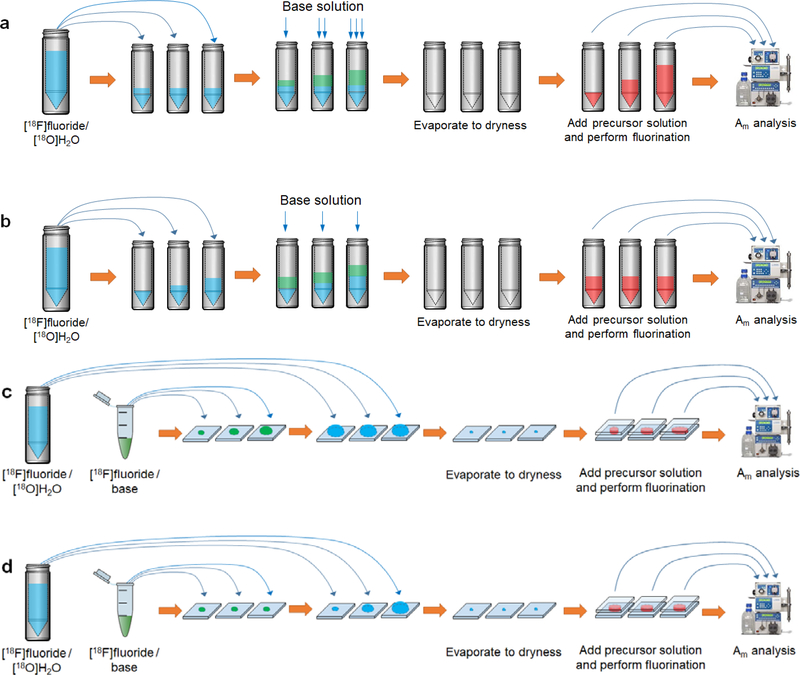
Figure 3: Experimental design for assessing the influence of variables on molar activity in macroscale and microscale radiosyntheses.
(a) Effect of reagent volume (macroscale). Equal aliquots of [18F]fluoride/[18O]H2O are added to reaction vials (to give equal starting radioactivity) followed by varying amounts of base solution. After evaporation to dryness, varying amounts of precursor (in proportion to base solution) are added and fluorination is performed. (b) Effect of starting radioactivity (macroscale). Varying amounts of [18F]fluoride/[18O]H2O are added to reaction vials (to give varying starting radioactivity) followed by equal amounts of base solution. After drying, equal amounts of precursor solution are added and fluorination is performed. (c) Effect of reaction droplet volume (microscale). Different combinations of [18F]fluoride/base solution and [18F]fluoride/[18O]H2O solution are added to chips such that starting radioactivity is matched but the amount of base varies. Following drying, varying amounts of precursor solution are added (in proportion to base) and fluorination is performed. (d) Effect of starting radioactivity (microscale). Equal amounts of [18F]fluoride/base and varying amounts of [18F]fluoride/[18O]H2O are added to chips such that starting radioactivity varies but amount of base is identical. Evaporation is performed and equal amounts of precursor solution are then added and fluorination is performed.