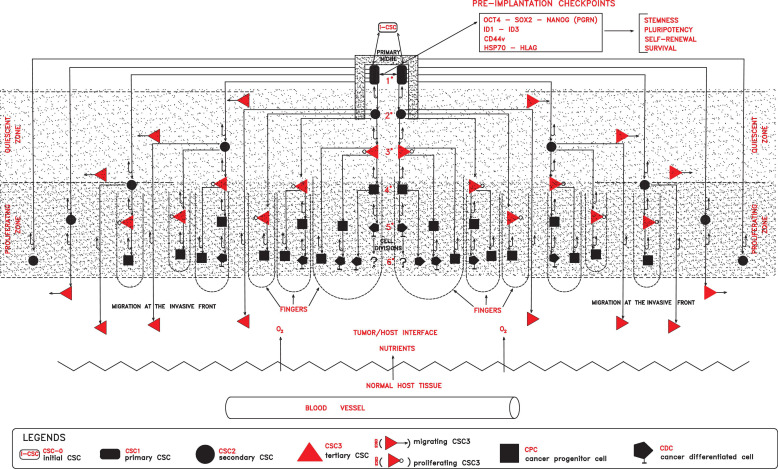
FIGURE 1.
In a primary niche, primary self-renewing cancer stem cells (CSC1s) endowed with stemness properties, due to defined preimplantation checkpoint factors (OCT4, SOX2, NANOG, ID, CD44, HSP70, and HLA-G), would generate progressively secondary proliferating CSCs (CSC2s), tertiary mesenchymal CSCs (CSC3s), cancer progenitor (CPCs), and cancer differentiated (CDCs) cells, globally forming a tumor module where two zones would lie (quiescent and proliferating). In the proliferating zone, more external with normoxia conditions, CSC3s and CPCs would proliferate, generating cell cord-finger structures on the invasive front at the tumor/host interface. On the other hand, in the quiescent zone, more internal with hypoxic conditions, quiescent CSC3s would be induced to migrate peripherally, seeding new local niches in the normal host tissues. All these processes, finally, would result in a tumor module with a defined cell heterogeneity and hierarchy.