Scheme 6. Proposed Mechanism for the Cu-Catalyzed Enantioselective Addition of Grignard Reagents to Substrate 1a.
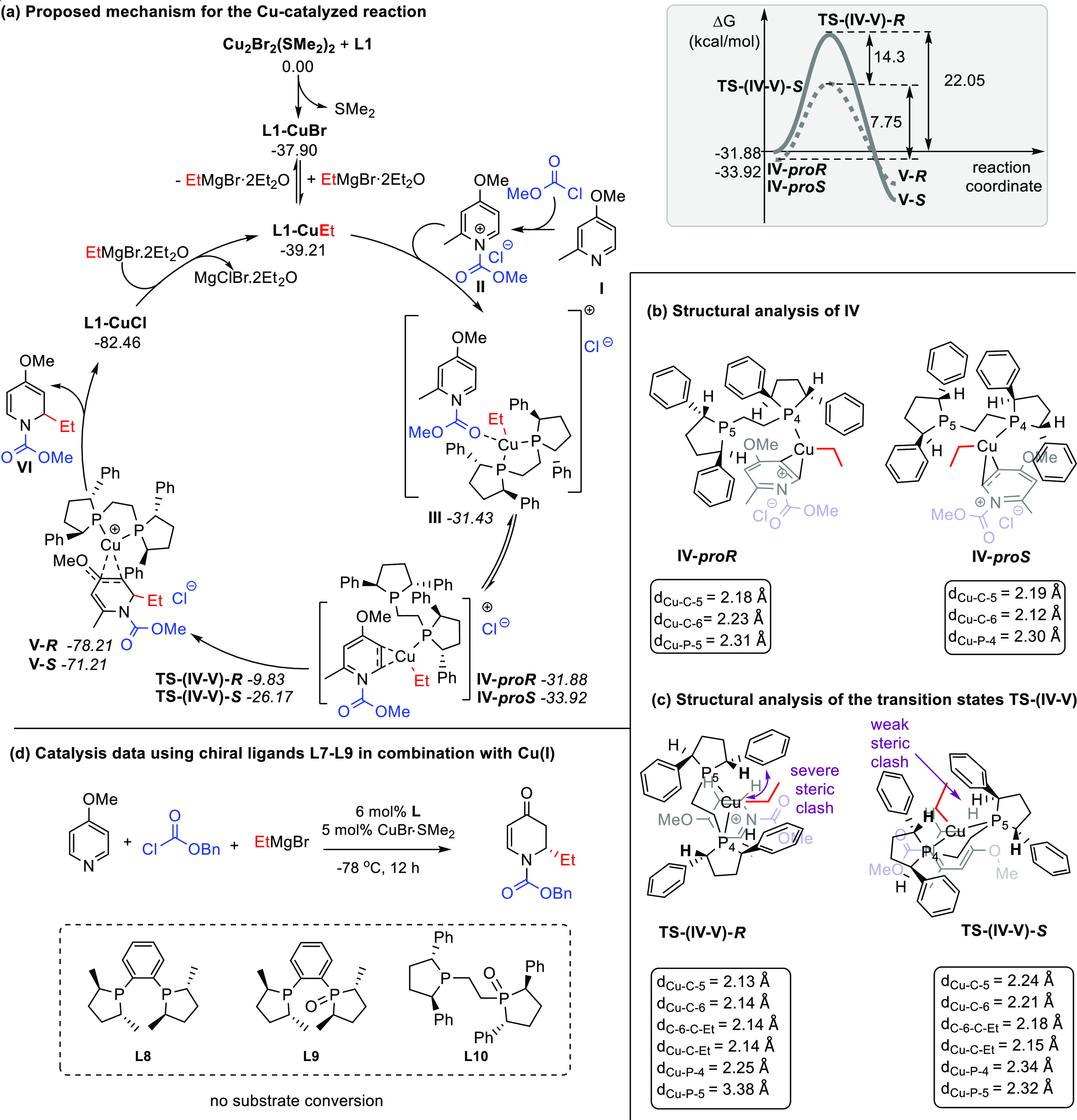
Calculations were performed at the PCM(CH2Cl2)12/B3LYP-D3/def2tzvpp//B3LYP-D3/def2svpp13 computational level using the Gaussian 09 program.14 The thermochemistry was obtained at 1 atm and 298.15 K. The pyridinium species and the L1 ligand are depicted in black, the protecting group in blue, and the ethyl group in red. (a) Left: proposed reaction mechanism. Right: reaction profile of the diastereoselective-determining step. (b) Structural analysis of the minima IV (proR and proS). (c) Structural analysis of the transition states TS-(IV-V). For visualization purposes, additional color coding is used in sections (b) and (c), showing the pyridinium species in light gray and blue, the L1 ligand in black, and the ethyl group in red. (d) Results of the catalytic addition of EtMgBr to 1a using Cu(I) salt in combination with chiral ligands L8–L10 under optimized reaction conditions.
