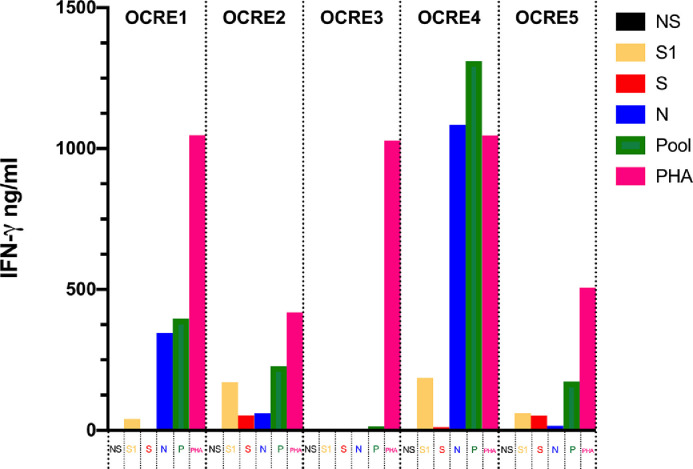
Fig. 1.
Interferon-gamma release assay for the detection of T-cell responses to SARS-CoV-2 in 5 multiple sclerosis patients treated with ocrelizumab.
T-cell specific response to SARS-CoV-2 was assessed with an IFN-γ release assay after overnight stimulation with SARS-CoV-2 peptide libraries (PepTivator® SARS-CoV-2 Prot_S1, Prot_S and Prot_N). PepTivator® SARS-CoV-2 Prot_S1 covers the N-terminal S1 domain of the spike protein (amino acids [aa] 1–692). PepTivator® SARS-CoV-2 Prot_S covers selected immunodominant sequence domains of the spike protein (aa 304–338, 421–475, 492–519, 683–707, 741–770, 785–802, and 885–1273). PepTivatorSARS-CoV-2 Prot_N covers the complete sequence of the N phosphoprotein of SARS-CoV-2.
For each subject, 6 conditions were set up: unstimulated (NS); stimulation with PepTivator® SARS-CoV-2 Prot_S1 [1µg/ml] (S1); stimulation with PepTivator® SARS-CoV-2 Prot_S [1µg/ml] (S); stimulation with PepTivator® SARS-CoV-2 Prot_N [1µg/ml] (N); stimulation with pooled peptides, S1 + S + N [1µg/ml] (P); stimulation with phytohemagglutinin 5µg/ml (PHA). After overnight stimulation, IFN-γ concentration (ng/ml) in supernatants was assessed with a commercial ELISA KIT. Each sample was tested in duplicate. A 7-point standard curve was included in each ELISA session. IFN-γ response was defined as peptide or PHA stimulated condition minus unstimulated condition.