Figure 4 |. MATE transporters NorM-NG and PfMATE belong to two major MATE subfamilies and mediate multidrug efflux.
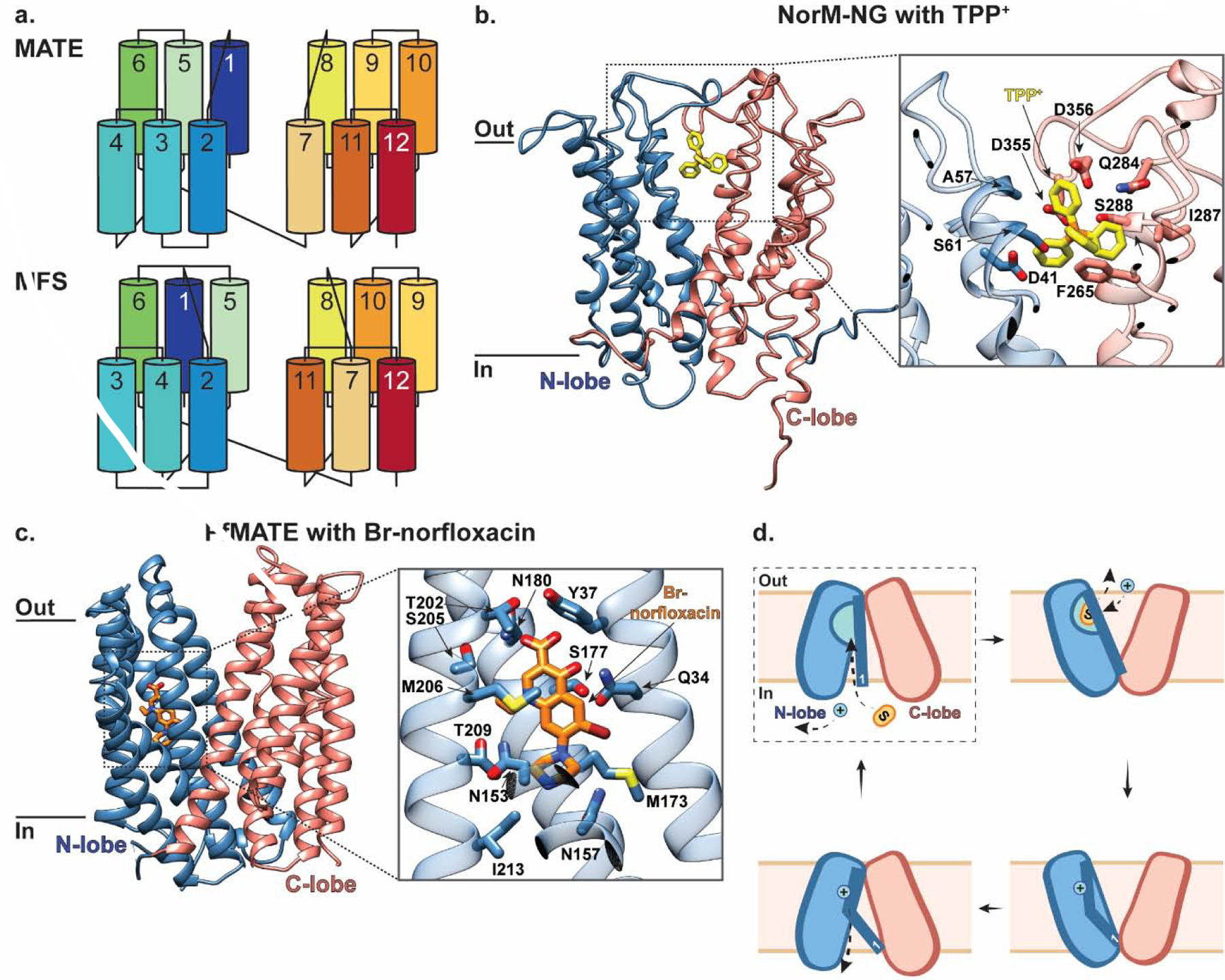
(a) Topology diagram comparing MATE and MFS transporters colored blue to red from the N- to C-terminus, adapted from [85] (b) The outward-facing structure of Norm-NG in the plane of the membrane. Inset shows a close-up of residues interacting with TPP+ (yellow; PDB ID: 4HUK). The N- and C- lobes are colored in blue and salmon, respectively, and the residues coordinating substrate are shown in stick representation. (c) The outward-facing structure of PfMATE, part of the DinF subfamily, in the plane of the membrane. Inset shows a close-up of residues interacting with Br-norflaxacin (orange; PDB ID: 3VVP). PfMATE is colored the same as NorM-NG in panel (b), and the residues coordinating substrate are shown in stick representation. (d) Schematic representation of PfMATE-mediated multidrug efflux. PfMATE switches between inward- and outward-facing conformations to provide an N-lobe localized binding pocket with alternating access to the cytosolic and extra-cytosolic solutions, respectively. In the inward-facing state, TM1 is largely kinked with D41 protonated (dashed box indicates this state is not yet structurally characterized). Deprotonation of D41 in this inward-facing state has been proposed to straighten TM1, which allows space for substrate to bind within the N-lobe cavity. This substrate binding induces a conformational switch to the outward-facing state with TM1 still straight. Here, D41 is re-protonated which causes TM1 to kink again and the N-lobe cavity to collapse, thus triggering substrate release and subsequent transition back to an inward-facing conformation.
