Figure 5. Conserved Nramp binding site changes across conformational states.
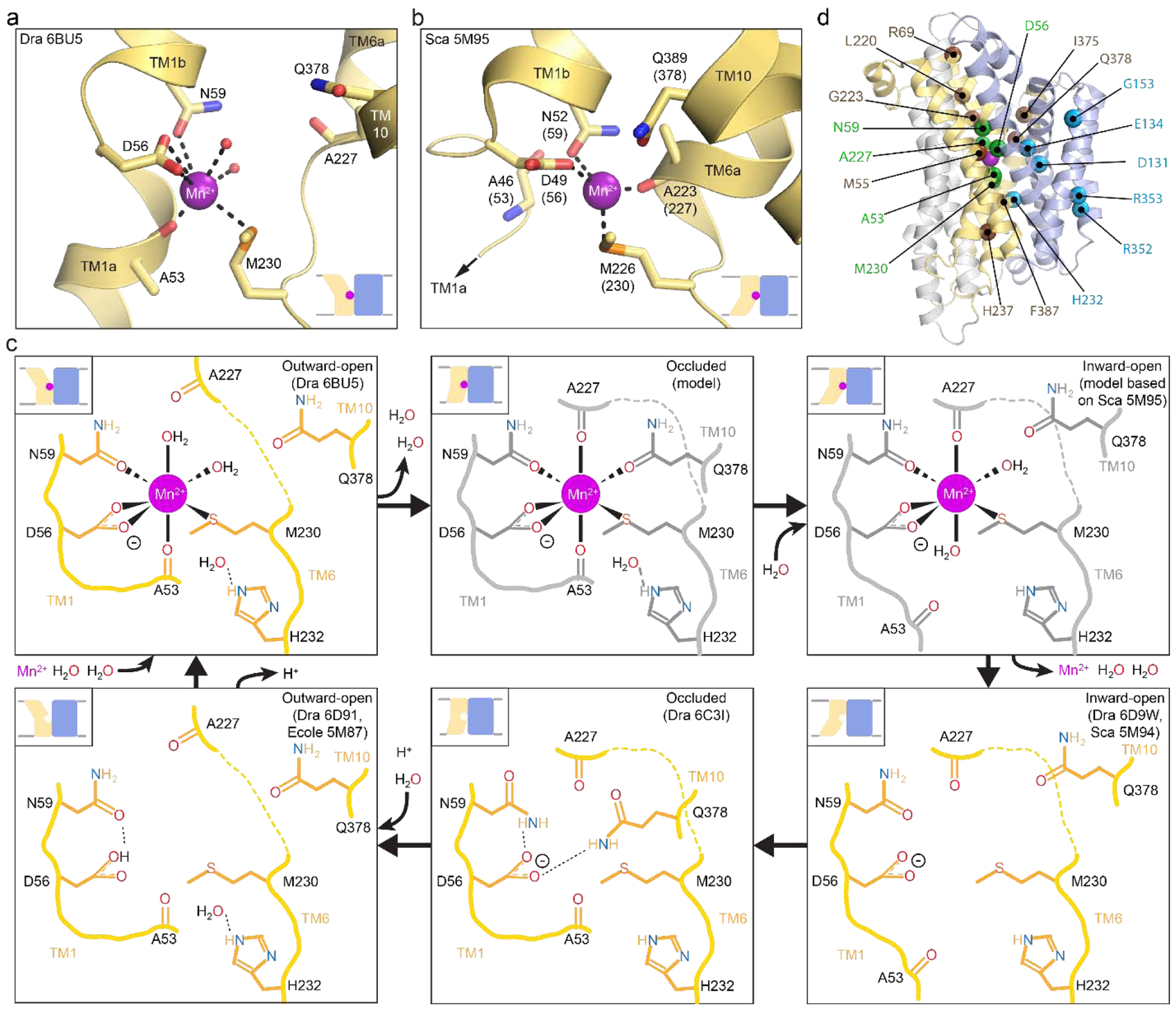
(a,b) Structures of outward-open DraNramp (a; PDB ID: 6BU5) and inward-open ScaNramp (b; PDB ID: 5M95; DraNramp residue numbering in parentheses) include bound Mn2+ substrate. In the outward-open DraNramp, D56, N59, M230 and the A53 carbonyl, along with two water molecules, coordinate Mn2+, while in the inward-open ScaNramp D56, N59, M230, and the A227 carbonyl coordinate Mn2+ (DraNramp residue numbering). (c) Model for changes in the metal-binding site during the transport cycle, including a possible switch from four to six to four metal-binding residues from the outward-open state, through a hypothetical occluded conformation (gray) in which Q378 may coordinate the metal, to the inward-open state. A change in metal coordination could serve to preferentially stabilize the conformational transition state to lower the activation energy barrier to transport. The surrounding binding-site residues may stabilize the negatively charged D56 during the return to the outward-open state, while D56 protonation likely then orients neighboring N59 to optimally bind incoming metal. Model adapted from Bozzi et al. 2019 [136]. (d) Mapped on the outward-open DraNramp structure are the Cα positions of residues from the inner metal coordination sphere (green), the outer selectivity sphere (brown) or the salt-bridge network (cyan) that influence metal selectivity, as discussed in the text.
