Abstract
As one of the most important post-translational modifications, glycosylation plays a pivotal role in many essential physiological functions, including cell recognition, signaling, and immune response. Thus, various qualitative and quantitative analytical strategies for glycomic profiling have been developed in recent decades. However, while extensive efforts have been devoted to the analysis of N-glycans, high throughput quantitative analysis of O-glycans is often overlooked and underexplored. This is partially due to the lack of universal enzyme for the release of O-glycans from protein backbone. Furthermore, the traditional chemical releasing method suffers from severe side reactions and involves tedious sample preparation procedures. Here, a multiplexed isobaric labeling method enabled by N, N-dimethyl leucine containing pyrazolone analog (DiLeuPMP) is introduced. This method combines the release and labeling of O-glycans in a one-pot reaction and achieves accurate MS2-based relative quantification with the ability to process four samples at a time. The method has been applied to core-1 O-glycan standard and three glycoproteins first and the results demonstrated its validity. Following this proof-of-principle demonstration, we analyzed more complex biological specimen using human serum samples. Overall, this method provides an effective and reliable approach for the profiling and high-throughput quantitative analysis of O-glycans in complex samples.
Keywords: isobaric labeling, quantitative glycomics, O-glycans, mass spectrometry, DiLeuPMP, MS2-based quantification
Graphical Abstract
INTRODUCTION
Protein glycosylation is a pivotal post-translational modification (PTM) and plays essential roles in many biological processes such as immune response, receptor recognition, cellular communication, and embryonic development1–3. The important biological functions and ubiquitous existence of glycosylated proteins in nature have made pressing needs for developing simple and sensitive platforms capable of analyzing glycans. Typically, glycosylation is classified into two types, N-linked and O-linked glycosylation, based on the attachment between oligosaccharides and amino acid residues. N-glycans conjugate to asparagine (N) residues in the consensus peptide motif of Asn-X-Ser/Thr (where X is any amino acid except proline) with a core structure (GlcNAc2Man3), while O-glycans are conjugated to serine (S) or threonine (T) without a clear consensus motif and consistent core structure.
For a typical glycomics analysis, cleavage of glycans from the peptide backbone is commonly the first step. For N-glycans, they can be readily released by enzymes such as PNGase F and PNGase A4. In contrast, the release and recovery of O-glycans remain to be a very challenging problem. Due to lack of a common core structure, O-glycans are typically categorized into eight subclasses (core-1 to core-8)5. Although endo-α-N-acetylgalactosaminidase (O-glycanase) is reported to be able to cleave the core-1 type O-glycans (Gal-GalNAc), there is no universal O-glycosidase that enables removal of all O-glycans. Thus, the chemical method so-called β-elimination, is often employed for releasing O-glycans in practice. β-elimination is performed under alkali conditions, and the strong base removes the β-proton on amino acid residue where an elimination reaction is triggered making for an unsaturated amino acid (from serine or threonine residues) together with the released O-glycans. However, this process can proceed stepwise and remove one saccharide residue at a time from the polysaccharide backbone, which results in the degradation of released glycans (Figure S1). This side reaction is known as ‘peeling’6–8. Previously, scientists found that adding blocking reagent 1-phenyl-3-methyl-5-pyrazolone (PMP) during β-elimination can suppress side reactions since PMP derivatization of the released O-glycans proceeds faster than peeling degradation and thus is able to competitively inhibit peeling process9–11. Mass difference-based isotopic labeling strategy using light and heavy isotopic PMP reagent was also reported to achieve quantitative glycomics analysis11, 12. However, the multiplexing capacity was limited to dual-plex due to the increase in spectral complexity and limited commercially available isotopic PMP reagents.
Mass spectrometry (MS) has emerged as a prominent analytical tool for the O-glycomics analysis13, 14. Currently, developed methods have largely improved the recovery and facilitated the profiling of O-glycans, but most of them suffer from low throughput and limited capacity for quantitative analyses. Furthermore, the procedures are usually time-consuming and tedious. Isobaric labeling is a popular approach in MS to achieve high throughput analysis based on MS2 fragmentation and reporter ion quantification. Our laboratory has previously developed a set of custom isobaric labeling reagents, N, N-dimethyl leucine (DiLeu), for large-scale quantitative proteomics and peptidomics15. Very recently, the general architecture of DiLeu was borrowed and extended for the development of a novel set of isobaric multiplex reagent for carbonyl-containing compound (SUGAR) tags capable of quantitative N-glycomics4. The SUGAR tags employed reductive amination chemistry on the reducing end of released N-glycans, thus are not applicable for direct analysis of O-glycans. Herein, we combined the isobaric labeling strategy employed by DiLeu with PMP-aided β-elimination to develop a novel chemical tool, 4-plex N, N-dimethyl leucine containing pyrazolone analog (DiLeuPMP), which provides the first isobaric tag aiming at high throughput quantitative O-glycan analysis and facilitates the application of O-glycomics.
EXPERIMENTAL SECTION
Materials and reagents
Acetic acid (AA), acetonitrile (ACN), N, N-dimethylformamide (DMF), tetrahydrofuran (THF), carbonyldiimidazole (CDI), tris-(2-carboxyethyl)phosphine (TCEP), formic acid (FA), methanol (MeOH), chloroform (CHCl3), 20% ammonium hydroxide (NH4OH) and water (HPLC grade) were purchased from Fisher Scientific (Pittsburgh, PA). 2-(4-aminophenyl)-5-methyl-2,4-dihydro-3H-pyrazole-3-one was purchased from Matrix Scientific (Columbia, SC). Core-1 O-glycan standard, bovine fetuin, porcine stomach mucin (PSM) and bovine submaxillary mucin (BSM) were purchased from Sigma-Aldrich (St. Louis, MO). Single donor healthy human serum was purchased from Innovative Research Inc. (Novi, MI). PNGase F was purchased from Promega (Madison, WI). Microcon-10kDa centrifugal filters (10K MWCO) were purchased from Merck Millipore Ltd. (Darmstadt, Germany). Sep-Pak C18 Cartridges were purchased from Waters Corporation (Milford, MA). Pierce™- C18 Tips, 10 ul bed were purchased from Thermo Fisher Scientific (Waltham, MA). Ethylene Bridged Hybrid C18 packing material (1.7 μm) was purchased from PolyLC Inc. (Columbia, MD). Fused silica capillary tubing (inner diameter 75 μm, outer diameter 375 μm) was purchased from Polymicro Technologies (Phoenix, AZ). All reagents were used without additional purification.
Synthesis of DiLeuPMP tags
L-Leucine and sodium cyanoborohydride (NaBH3CN) were suspended in MeOH and the mixture was cooled in an ice-water bath. Then, formaldehyde (CH2O, 37% w/w) was added dropwise, and the mixture was stirred in an ice-water bath for 30 min. The product was purified by flash column chromatography (MeOH/DCM) and dried in vacuo to produce white solid N, N-dimethyl leucine (DiLeu). The DiLeu reagent was then dissolved in anhydrous THF with 1.2 molar excess of carbonyldiimidazole (CDI) at room temperature for 30 min and then equal molar of 2-(4-aminophenyl)-5-methyl-2,4-dihydro-3H-pyrazole-3-one was added. The reaction was heated to 70°C for 18hr and the non-isotopic version of DiLeuPMP was purified by flash column chromatography (MeOH/DCM) as brown color oil. The isobaric version of 4-plex DiLeuPMP reagents were synthesized following the same procedures with different isotopic starting reagents (Figure S2). For the channels requiring 18O exchange, isotopic leucine was dissolved in 1 N HCl H218O solution (pH 1) and stirred on a hot plate at 65 °C for 4 h. Following evaporation of HCl from the solution in vacuo, trace amounts of acid were removed with StratoSpheres PL-HCO3 MP resin (Agilent Technologies) to obtain 18O L-leucine in free base form. The purity was tested using HPLC and structures were confirmed by NMR and/or MS (Figures S3 – S5).
DiLeuPMP labeling on core-1 O-glycan standard
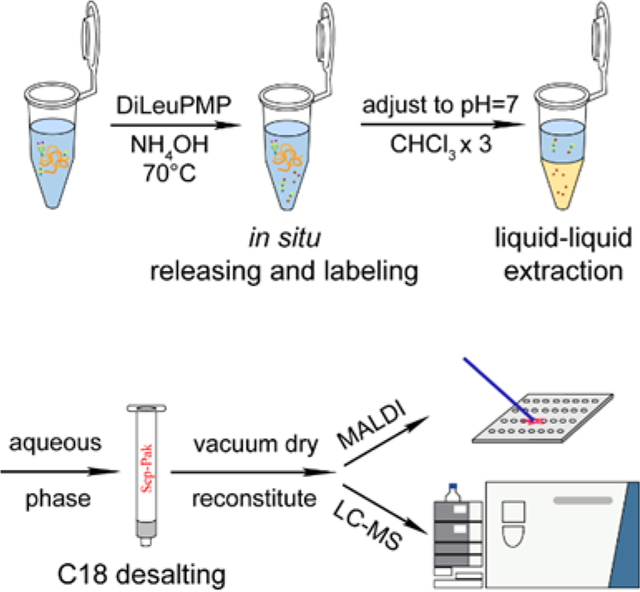
The core-1 O-glycan standard was first dissolved in deionized water to make a stock solution at 1 mg/mL. 0.5 M DiLeuPMP was prepared in MeOH/NH4OH (50/50 v/v) at final concentration of 10% NH4OH. 10 μL of core-1 standard and DiLeuPMP were equally mixed, and the reaction was stood at 70 °C for 1h. Then the mixture was desalted using Pierce™- C18 tips, and the eluted fractions were dried in vacuo, reconstituted in 20 μL of 5% ACN, and analyzed by MALDI-MS or LC-MS/MS immediately.
DiLeuPMP labeling on standard glycoproteins and human serum
The release of O-glycans from glycoproteins was performed according to the published procedure with moderate modifications16. Briefly, 100 μg of standard glycoproteins were dissolved in 10% NH4OH/DMF (50/50 v/v), and DiLeuPMP was added to make the final concentration at 0.5 M. After incubating at 70°C for 24h, the pH of the solution was adjusted to neutral by acetic acids followed by liquid extraction using chloroform for three times. Organic phase was discarded and remaining aqueous phase was desalted using Sep-Pak C18 Cartridge. The eluted fractions were dried in vacuo and reconstituted in 20 μL of 5% ACN, analyzed by MALDI-MS or LC-MS/MS. For 4-plex DiLeuPMP labeled samples, they were combined firstly before extraction.
In terms of human serum sample preparation, we followed FASP protocol with minor modifications.17 In brief, 100 μL healthy human serum was mixed with 300 μL 0.1% SDS and 100 mM TCEP in 10 mM sodium phosphate (pH 7.5) at 60 °C for 1 h. After the sample was cooled down, 10 kDa MWCO was used for buffer exchange. 400 μL of 10 mM sodium phosphate was used to rinse the filter three times, following additional three times of 0.5 M TEAB buffer exchange. 8U of PNGase F in 100 μL TEAB buffer was then added onto the filter and the sample was incubated at 37°C for 18 h to release and remove N-glycans. 10 kDa MWCO filters were used to separate proteins and glycans. After the proteins were recollected, ZIC-HILIC were applied for O-glycoprotein/peptide enrichment18. HILIC beads were first activated with 200 μL of elution buffer (0.1% TFA, 99.9% H2O) for 30 min and then washed with binding buffer (0.1% TFA, 19.9% H2O, 80% ACN) twice. Proteins were dissolved in 300 μL of binding buffer and mixed with 7 mg activated ZIC-HILIC resin at a 1:50 peptide-to-material mass ratio in a microcentrifuge tube. The tube was shaken over a vortex mixer for 1 h and the supernatant was removed by centrifugation. The beads were washed with 70 μL binding buffer three times and glycoprotein/peptides were eluted with 70 μL elution buffer. Same labeling procedure with DiLeuPMP was conducted subsequently as mentioned above.
Matrix-assisted Laser Desorption/Ionization (MALDI)-MS analysis
Samples were prepared by premixing 1 μL of DiLeuPMP-labeled O-glycans with 1 μL 2,5-dihydroxybenzoic acid (DHB) matrix (100 mg/mL in 5% N, N-dimethylaniline, 47.5% MeOH and 47.5% water), and 1 μL of each matrix/sample mixture was spotted onto the MALDI target plate. A MALDI-LTQ-Orbitrap XL mass spectrometer (Thermo Scientific, Bremen, Germany) was used for MALDI-MS analysis. Ionization was performed using laser energy of 17 μJ. Spectra were acquired in the Orbitrap mass analyzer within a mass range of m/z 1,000–4,000 at a mass resolution of 60 K (at m/z 400).
LC-MS/MS analysis
A self-fabricated nano-C18 column (15 cm, 75 μm i.d., 1.7 μm Ethylene Bridged Hybrid C18 packing material) was used for glycan separation. A Dionex Ultimate 3000 nanoLC system was coupled to a Q Exactive HF Hybrid Quadrupole Orbitrap Mass Spectrometer (Thermo Scientific, Bremen, Germany) for all LC-MS/MS analyses. Mobile phase A was water with 5% DMSO, and mobile phase B was ACN with 5% DMSO. The flow rate was set at 0.3 μL/min, and the injection volume was 2 μL. The following gradient was used (time, % mobile phase B) unless otherwise specified: (0 min, 5%), (28 min, 5%), (38 min, 9%), (128 min, 37%), (133 min, 95%), (143 min, 95%), (148 min, 5%), (158 min, 5%).
The following mass spectrometer parameters were used for all data acquisition. Samples were ionized in positive ion mode with a spray voltage of 3 kV. S-lens radio frequency (RF) level was set to be 30, and capillary temperature was set to be 300 °C. Full MS scans were acquired at m/z 350–2000 with resolving power of 120 K. Maximum injection time of 100 ms, automatic gain control (AGC) target value of 5×105, and 1 microscan were used for full MS scans. Top 15 data-dependent MS2 analysis was performed at a resolving power of 15 K with higher-energy collisional dissociation (HCD) operating with normalized collision energy (NCE) of 25. The first fixed mass sets to 100 m/z in order to obtain reporter ions. The isolation window was set as 2.5 m/z and the dynamic exclusion of acquired precursors was set to 15 sec with a ± 20 ppm tolerance.
O-glycan data analysis
The raw data was compared against an in-house database including the most possible combinations of O-glycan units (Hexose (H), HexNAc (N), Fucose (F), and NeuAc (S)). DiLeuPMP-labeled O-glycans were identified by accurate mass matching in full MS with a mass tolerance of 10 ppm and fragmentation matching in MS/MS spectra assisted by GlycoWorkbench. Peak areas of reporter ions for DiLeuPMP-labeled glycans were used for relative quantification. Microsoft Excel and Origin were used for calculations and statistical analyses.
RESULTS AND DISCUSSION
Numerous challenges exist for O-glycan analysis. In contrast to N-glycosylation, O-glycosylation lacks a known amino acid consensus sequence and Mucin-type O-glycosylation is usually highly heterogeneous. However, among the various challenges, the major difficulty lies in the lack of universal enzymatic tools for release of O-glycans from proteins. Therefore, the chemical method is currently the most effective and reliable way of acquiring released O-glycans, where β-elimination is the most common one. However, β-elimination demands alkaline conditions and usually results in degradation which was referred as “peeling”. Previously, researchers found that adding PMP can efficiently block the reducing end of released glycans and prevent them from sequential peeling degradation. The derivatization reaction itself is a Michael addition involving a two-step labeling process in which Michael donor molecules of the labeling reagent are formed and consecutively added to the reducing end of the glycan with a stoichiometry of two label molecules per glycan19. The use of PMP and related tags was restricted in fluorescence detection initially20 and was recently employed in the field of mass spectrometry10, 21, 22.
O-glycans are known to be associated with many critical biological functions. Abnormal O-glycosylation has been implicated in a variety of diseases, including familial tumoral calcinosis23, Tn syndrome24, and IgA nephropathy25. Thus, global profiling and quantitative analysis of O-glycans is crucial to understand the structures and functions of O-glycosylation in the development of these diseases. The current analytical tool suffers from low throughput and tedious sample preparation procedures. Mass spectrometry (MS)-based, isobaric labeling strategy for relative quantification allows for parallel multiplexing of experiments, which provides an opportunity to address these limitations for O-glycan analysis. Generally, multiple samples are chemically labeled with isobaric chemical tag variants and each variant has the same chemical structure and nominal mass which is indistinguishable in full MS spectrum. However, each variant is fragmented to produce a unique “reporter ion” during tandem MS and the signal intensity can be used for relative quantification. Commercially available reagents such as isobaric tags for relative and absolute quantification (iTRAQ) and tandem mass tags (TMT) are widely used in quantitative proteomics26. In our lab, we previously developed DiLeu isobaric tags for cost-effective proteomics27 and isobaric multiplex reagents for carbonyl-containing compound (SUGAR) tags for N-glycan analysis4, 28.
By combining the PMP labeling and DiLeu isobaric labeling strategies together, we designed and synthesized a set of 4-plex DiLeuPMP tags to enable high-throughput quantitative O-glycomics (Figure 1). The reactive site of Michael donor PMP is on the five-member ring. Hence, we incorporated DiLeu reporter ion onto the benzene moiety. DiLeuPMP was created in a two-step synthesis with an overall yield of 70%. The 4-plex isobaric version of DiLeuPMP tags were synthesized using heavy isotope-coded starting materials accordingly (Figure S2). The isotope purities of 4-plex DiLeuPMP were over 98% (Figure S3). As two label molecules were added per glycan, the chromatographic properties of the derivatized oligosaccharides were largely affected by the tags, thus we avoided incorporating deuterium atom into 4-plex DiLeuPMP structures, using only 13C, 15N, and 18O, to prevent possible retention time shift.
Figure 1.
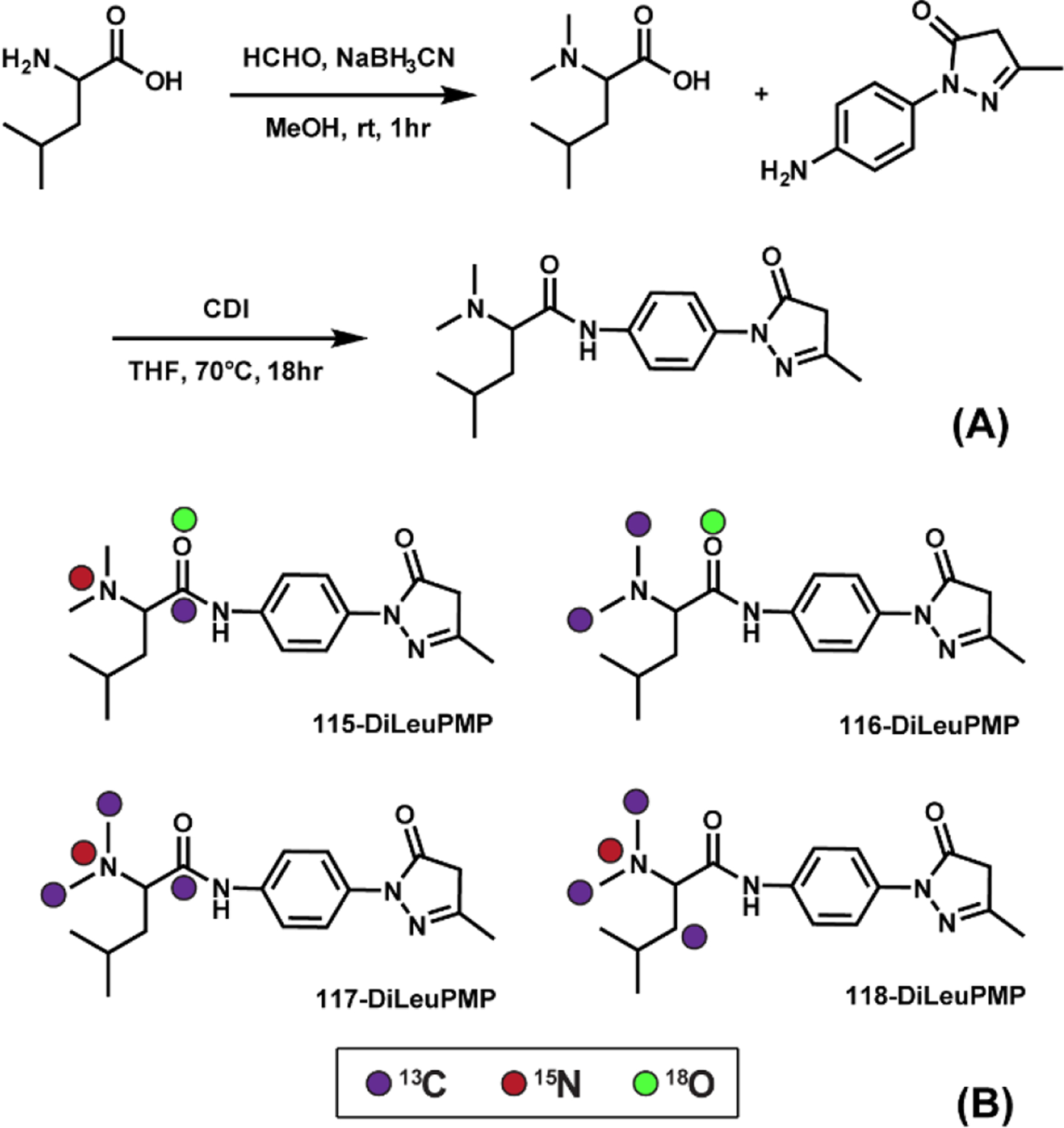
(A) Structure and synthetic routes of DiLeuPMP tag; (B) Isotopic configurations of 4-plex DiLeuPMP tags.
Releasing and labeling of O-glycans was performed in a one-pot manner. The general workflow is illustrated in Figure 2. After in situ releasing and labeling of O-glycans during β-elimination, the pH of the system was adjusted to neutral, and chloroform was used for liquid-liquid extraction. The organic layer was discarded to get rid of excess tags and the remaining aqueous phase was desalted using Sep-Pak C18 cartridges. The elute was dried and reconstituted for MALDI-MS or LC-MS/MS analysis. Compared to developed methods29, 30, we further lowered the alkalinity of the system, choosing 5% NH4OH instead of 100 mM NaOH or 50% hydrazine and found that in 5% NH4OH, the releasing of O-glycans was still efficient and the peeling degradation can be effectively suppressed.
Figure 2.
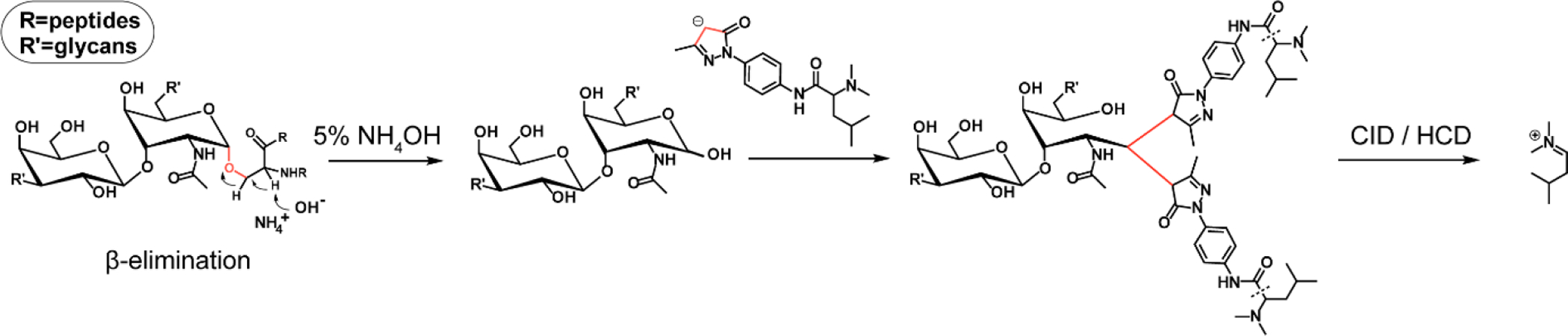
Scheme of released O-glycan labeled by DiLeuPMP at 2:1 (tag to glycan) ratio.
*The reaction site is highlighted in red
Commercially available core-1 O-glycan standard was used for method validation. As shown in Figure 3, after labeling, the signal corresponding to bis-DiLeuPMP labeled glycan (m/z 1026.5495) was abundant, while mono-DiLeuPMP labeled (m/z 696.3434) and peeling product (m/z 864.4965) were also observed. However, they were less than 5% of overall signal intensity, which indicated satisfactory labeling efficiency of DiLeuPMP and its efficient peeling suppression. It is worth mentioning that because two tags are added per glycan and it is the tag that commonly carries charges, on ESI-MS spectrum, the dominant peak corresponding to glycan conjugates carries +2 charges (m/z 513.7766 [M+2H]2+, m/z 524.7674 [M+H+Na]2+) while +1 charged signals are also noticeable (m/z 1026.5466 [M+H]+, m/z 1048.5283 [M+Na]+). The elevated charge states facilitate the fragmentation process and produce abundant fragment ions for structural elucidation.
Figure 3.
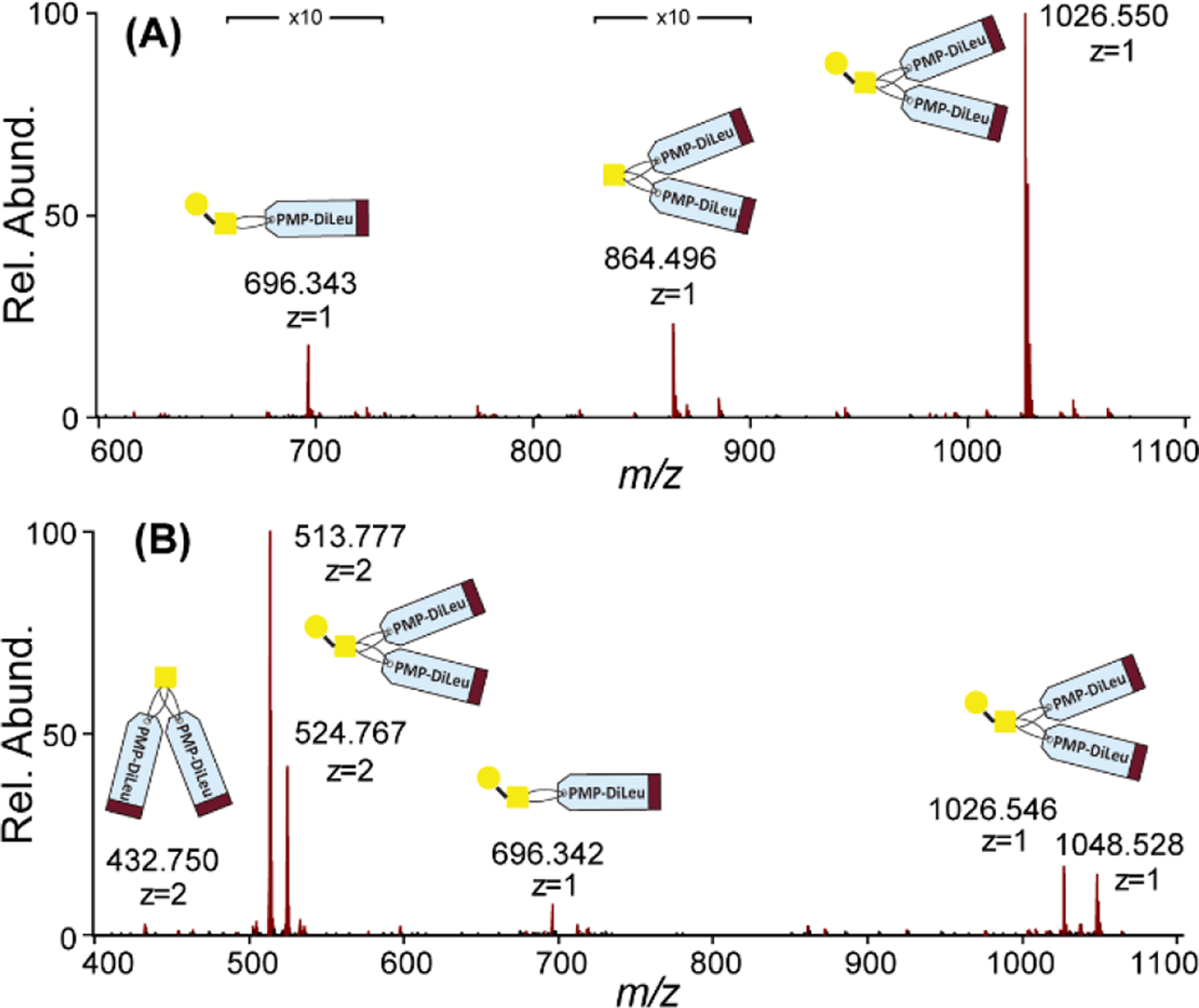
(A). MALDI-MS spectrum of DiLeuPMP labeled core-1 O-glycan standard; (B) ESI-MS spectrum of DiLeuPMP labeled core-1 O-glycan standard.
Various model glycoproteins were used to further evaluate the feasibility of the described method for O-glycan analysis on glycoproteins. Bovine fetuin, bovine submaxillary mucin (BSM) and porcine stomach mucin (PSM) were chosen as model glycoproteins as the O-glycans on these glycoproteins are well studied4, 28. Bovine fetuin, which contains both N- and O-linked glycans, has a relatively simple O-glycosylation pattern. BSM belongs to the class of salivary glycoproteins, and it consists of a long protein chain with numerous disaccharide and oligosaccharide side chains, some of which are known to be sialylated. PSM is primarily composed of carbohydrate units with protein contributing to only 20% of the molecular weight. Following the one-pot procedure described above, the O-glycans were released from these glycoproteins and labeled by DiLeuPMP. We set a control group using PMP and compared our results with previously established methods. As shown in Figure 4, for MALDI-MS profiling, 3 O-glycans were found on bovine fetuin, 14 O-glycans were identified on BSM and 26 O-glycans were identified on PSM using DiLeuPMP labeling. Our results showed great overlap between PMP control as expected and the identified O-glycans were similar to those previously reported31, 32 (Figure S6, Table S1). After coupling with LC separation, a higher number of identifications and coverage were achieved in LC-MS/MS results (Table S2). The distinct profiling, high coverage, and good reproducibility of identified glycans between PMP and DiLeuPMP demonstrated the consistency and robustness of our new method compared with existing methods.
Figure 4.
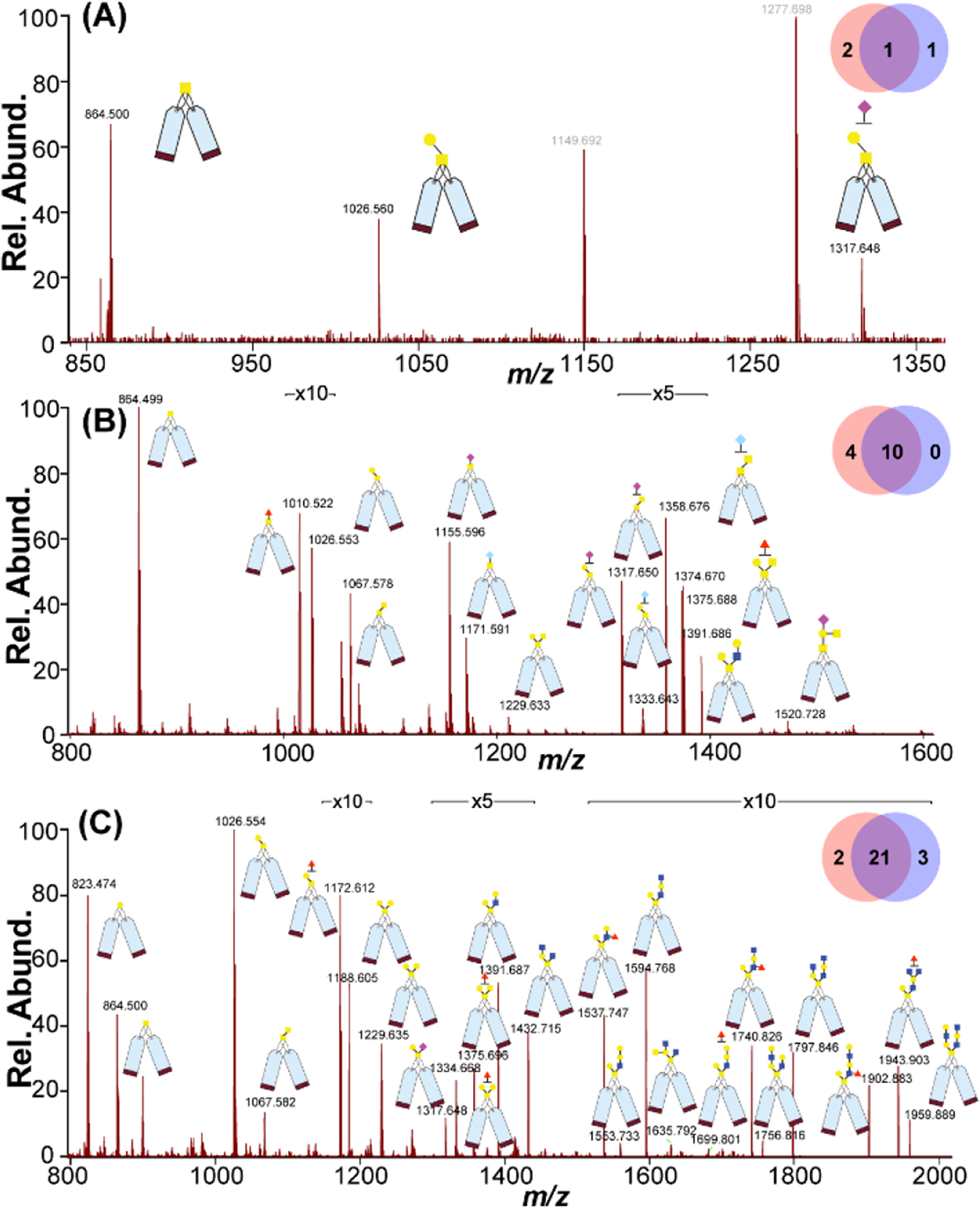
MALDI-MS profiling of DiLeuPMP labeled O-glycans released from standard glycoproteins (A) Bovine fetuin; (B) Bovine Submaxillary Mucin (BSM); (C) Porcine Stomach Mucin (PSM). The inset Venn diagrams above each spectrum show the numbers of identified glycans from PMP control (blue purple, right) and DiLeuPMP (orange red, left).
The quantification performance of the 4-plex DiLeuPMP tags was evaluated next by labeling O-glycans on PSM at known ratios. Denatured PSM was aliquoted into four equal portions in triplicate and labeled with 4-plex DiLeuPMP tags respectively, and then they were mixed with known ratios at 1:1:5:10 before performing liquid extraction and subsequent desalting. The combined desalted samples were then vacuum dried and reconstituted before performing LC-MS/MS analysis. The intensities of reporter ions in MS/MS spectra for each conjugate were used to calculate the experimental ratios. In Figure 5A, experimental ratios of 4-plex DiLeuPMP-labeled O-glycans are plotted against theoretical ratios 1:1:5:10. Representative MS2 spectra of low mass range containing reporter ions are shown in Figure 5B. For all three known ratios, less than 15% relative errors were observed with standard deviations of 0.15, 0.19, and 0.23, demonstrating that the DiLeuPMP quantification approach offers an accurate tool for quantitative analysis of O-glycans in a high-throughput manner. Moreover, no retention time shift was observed for 4-plex DiLeuPMP tag labeled O-glycans on the C18 column, as shown in Figure 5C.
Figure 5.
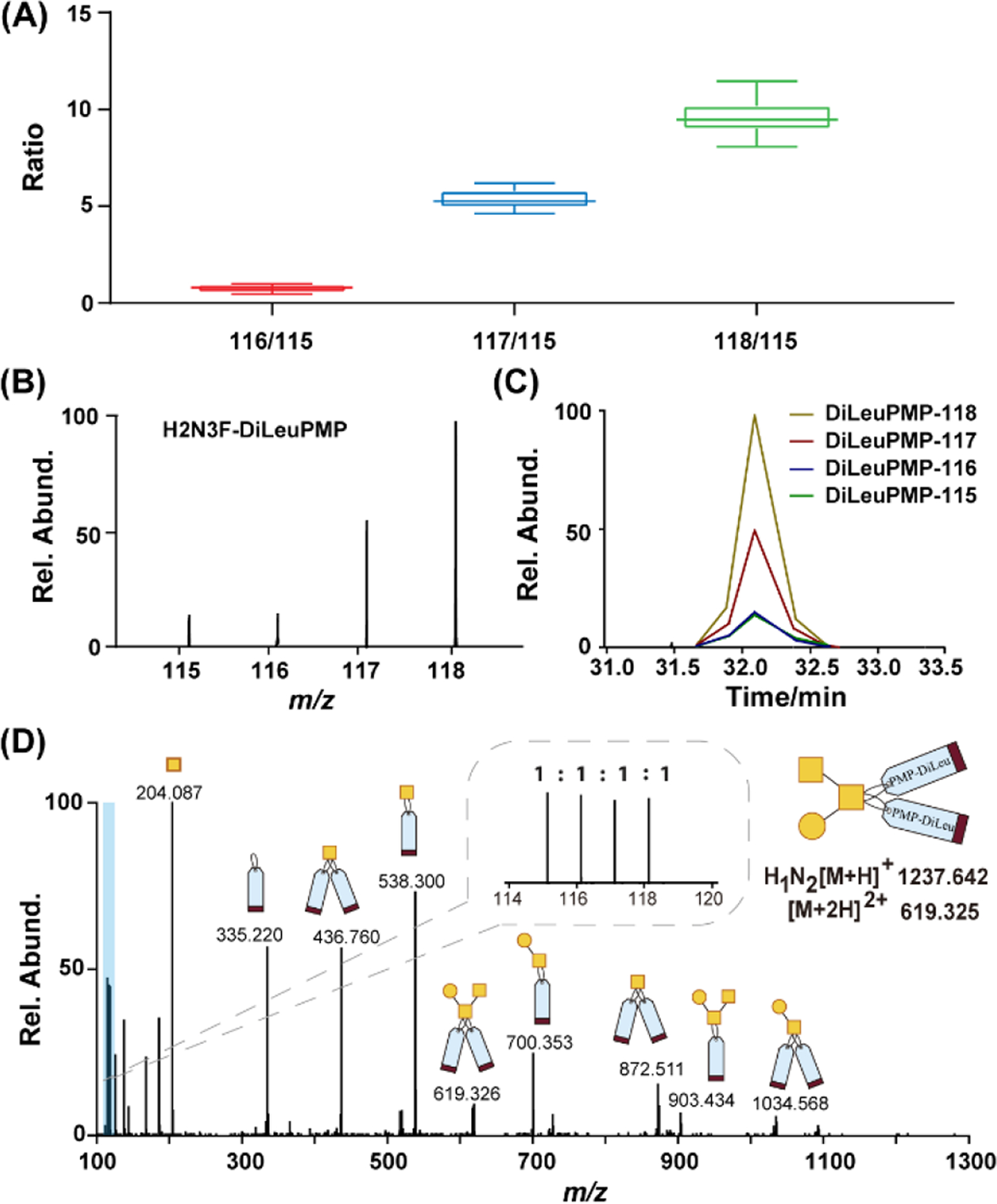
(A) Relative quantification performance of 4-plex DiLeuPMP labeled O-glycans released from glycoprotein PSM. Labeled O-glycans were mixed at ratios of 1:1:5:10 and analyzed in triplicates. Box plots show the median (line), the 25th and 75th percentile (box), and the 5th and 95th percentile (whiskers); (B) Representative MS spectrum of reporter ion region for 4-plex DiLeuPMP labeled H2N3F; (C) LC retention times of reporter ions generated from 4-plex DiLeuPMP labeled H2N3F; (D) ESI-MS/MS fragmentation of DiLeuPMP labeled H1N2 from healthy human serum sample showing efficient fragmentation and abundant reporter ions in the zoomed inset allowing quantification. Note: H-hexose, N-N-acetylhexoamine, F-fucose.
Lastly, we applied our DiLeuPMP labeling strategy to the O-glycan analysis of healthy human serum for exploration in real biological samples. Since numerous clinically relevant analyses utilize human blood serum or plasma for both routine clinical measurements and potential disease biomarker discovery, the ability to analyze real biological specimen will surely expand the scope of usage of DiLeuPMP tags. The healthy human serum was collected at a FDA-approved collection center. The sample preparation was followed by a previously reported method with moderate modifications33. Briefly, the proteins were extracted and denatured firstly, and N-glycans were removed prior to O-glycoprotein/peptides enrichment. The enriched samples were then aliquoted into four equal portions before being labeled by 4-plex DiLeuPMP. After the derivatization procedure, four samples were equally mixed followed by cleanup procedure prior to LC-MS analysis. The data analysis revealed that 12 O-glycans could be reliably recorded in a profile with a quantitative ratio error of less than 15% (Table S3). We anticipate that these are among the most abundant O-glycans in human serum, and it is consistent with previous studies33, 34. Furthermore, it is noted that the fragment ions from the precursors are abundant, which aids manual confirmation and structural elucidation of O-glycans (Figure 5D and Figure S7). In summary, our results shed light on the potential application of the DiLeuPMP labeling strategy for high-throughput quantitative O-glycomic analysis of clinical samples.
CONCLUSIONS
In summary, 4-plex DiLeuPMP isobaric tags were developed in this study for quantitative O-glycomics. This is the first isobaric tag designed and developed for high-throughput quantitative analysis of O-glycans. By combining traditional PMP-aided β-elimination method and DiLeu enabled isobaric labeling strategy together, DiLeuPMP achieved releasing and labeling of O-glycans simultaneously as well as enabling high throughput MS2-based quantitative analysis. The labeling efficiency of DiLeuPMP is high and the peeling degradation is suppressed in a decreased alkali condition. The labeling pattern of two tags per glycan elevated the charges of labeled glycan conjugates and thus facilitated the fragmentation during MS/MS and produced abundant fragment ions for structural elucidation and reporter ions for quantification. We also demonstrated accurate relative quantification across an order of magnitude dynamic range using standard glycoproteins with these novel isobaric tags and applied the method to the analysis of human serum sample, suggesting the potential applications for large-scale analyses of biological and clinical specimens. Overall, the successful development of DiLeuPMP tags offers a powerful chemical tool for glycomics study in many biological and clinical applications and makes parallel profiling and quantitative analysis of O-glycans readily implemented. In conclusion, we anticipate that the novel DiLeuPMP labeling approach can be widely applied in a variety of biomedical research areas.
Supplementary Material
ACKNOWLEDGMENTS
Support for this research was provided in part by the NIH grants U01CA231081, R01DK071801, RF1AG052324, and P41GM108538. The Orbitrap instruments were purchased through the support of an NIH shared instrument grant (NIH-NCRR S10RR029531) and the University of Wisconsin-Madison, Office of the Vice Chancellor for Research and Graduate Education with funding from the Wisconsin Alumni Research Foundation. LL acknowledges a Vilas Distinguished Achievement Professorship and Charles Melbourne Johnson Professorship with funding provided by the Wisconsin Alumni Research Foundation and University of Wisconsin-Madison School of Pharmacy.
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website
Figures S1-S7 and Tables S1-S3
REFERENCES
- 1.Dell A; Morris HR, Glycoprotein structure determination mass spectrometry. Science 2001, 291 (5512), 2351–2356. [DOI] [PubMed] [Google Scholar]
- 2.Helenius A; Aebi M, Intracellular functions of N-linked glycans. Science 2001, 291 (5512), 2364–2369. [DOI] [PubMed] [Google Scholar]
- 3.Marth JD; Grewal PK, Mammalian glycosylation in immunity. Nat. Rev. Immunol 2008, 8 (11), 874–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feng Y; Li MY; Lin YY; Chen BM; Li LJ, Multiplex Quantitative Glycomics Enabled by Periodate Oxidation and Triplex Mass Defect Isobaric Multiplex Reagents for Carbonyl-Containing Compound Tags. Anal. Chem 2019, 91 (18), 11932–11937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jensen PH; Kolarich D; Packer NH, Mucin-type O-glycosylation - putting the pieces together. FEBS J. 2010, 277 (1), 81–94. [DOI] [PubMed] [Google Scholar]
- 6.Mulagapati S; Koppolu V; Raju TS, Decoding of O-Linked Glycosylation by Mass Spectrometry. Biochemistry 2017, 56 (9), 1218–1226. [DOI] [PubMed] [Google Scholar]
- 7.Huang YP; Mechref Y; Novotny MV, Microscale nonreductive release of O-linked glycans for subsequent analysis through MALDI mass spectrometry and capillary electrophoresis. Anal. Chem 2001, 73 (24), 6063–6069. [DOI] [PubMed] [Google Scholar]
- 8.Goetz JA; Novotny MV; Mechref Y, Enzymatic/Chemical Release of O-Glycans Allowing MS Analysis at High Sensitivity. Anal. Chem 2009, 81 (23), 9546–9552. [DOI] [PubMed] [Google Scholar]
- 9.Furukawa J; Fujitani N; Araki K; Takegawa Y; Kodama K; Shinohara Y, A Versatile Method for Analysis of Serine/Threonine Posttranslational Modifications by beta-Elimination in the Presence of Pyrazolone Analogues. Anal. Chem 2011, 83 (23), 9060–9067. [DOI] [PubMed] [Google Scholar]
- 10.Wang CJ; Fan WC; Zhang P; Wang ZF; Huang LJ, One-pot nonreductive O-glycan release and labeling with 1-phenyl-3-methyl-5-pyrazolone followed by ESI-MS analysis. Proteomics 2011, 11 (21), 4229–4242. [DOI] [PubMed] [Google Scholar]
- 11.Sic S; Maier NM; Rizzi AM, Quantitative fingerprinting of O-linked glycans released from proteins using isotopic coded labeling with deuterated 1-phenyl-3-methyl-5-pyrazolone. J. Chromatogr. A 2015, 1408, 93–100. [DOI] [PubMed] [Google Scholar]
- 12.Zhang P; Zhang Y; Xue XD; Wang CJ; Wang ZF; Huang LJ, Relative quantitation of glycans using stable isotopic labels 1-(d0/d5) phenyl-3-methyl-5-pyrazolone by mass spectrometry. Anal. Biochem 2011, 418 (1), 1–9. [DOI] [PubMed] [Google Scholar]
- 13.Zauner G; Kozak RP; Gardner RA; Fernandes DL; Deelder AM; Wuhrer M, Protein O-glycosylation analysis. Biol. Chem 2012, 393 (8), 687–708. [DOI] [PubMed] [Google Scholar]
- 14.Wilkinson H; Saldova R, Current Methods for the Characterization of O-Glycans. J. Proteome Res 2020, 19 (10), 3890–3905. [DOI] [PubMed] [Google Scholar]
- 15.Xiang F; Ye H; Chen RB; Fu Q; Li LJ, N,N-Dimethyl Leucines as Novel Isobaric Tandem Mass Tags for Quantitative Proteomics and Peptidomics. Anal. Chem 2010, 82 (7), 2817–2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karlsson NG; Packer NH, Analysis of O-linked reducing oligosaccharides released by an in-line flow system. Anal. Biochem 2002, 305 (2), 173–85. [DOI] [PubMed] [Google Scholar]
- 17.Ni MW; Wang L; Chen W; Mou HZ; Zhou J; Zheng ZG, Modified filter-aided sample preparation (FASP) method increases peptide and protein identifications for shotgun proteomics. Rapid Commun. Mass Spectrom 2017, 31 (2), 171–178. [DOI] [PubMed] [Google Scholar]
- 18.Yu Q; Wang B; Chen Z; Urabe G; Glover MS; Shi X; Guo LW; Kent KC; Li L, Electron-Transfer/Higher-Energy Collision Dissociation (EThcD)-Enabled Intact Glycopeptide/Glycoproteome Characterization. J. Am. Soc. Mass Spectrom 2017, 28 (9), 1751–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ruhaak LR; Zauner G; Huhn C; Bruggink C; Deelder AM; Wuhrer M, Glycan labeling strategies and their use in identification and quantification. Anal. Bioanal. Chem 2010, 397 (8), 3457–3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goso Y; Sugaya T; Ishihara K; Kurihara M, Comparison of Methods to Release Mucin-Type O-Glycans for Glycomic Analysis. Anal. Chem 2017, 89 (17), 8870–8876. [DOI] [PubMed] [Google Scholar]
- 21.Wang CJ; Lu Y; Han JL; Jin WJ; Li LM; Zhang Y; Song XZ; Huang LJ; Wang ZF, Simultaneous Release and Labeling of O- and N-Glycans Allowing for Rapid Glycomic Analysis by Online LC-UV-ESI-MS/MS. J. Proteome Res 2018, 17 (7), 2345–2357. [DOI] [PubMed] [Google Scholar]
- 22.Yuan JB; Wang CJ; Sun YJ; Huang LJ; Wang ZF, Nonreductive chemical release of intact N-glycans for subsequent labeling and analysis by mass spectrometry. Anal. Biochem 2014, 462, 1–9. [DOI] [PubMed] [Google Scholar]
- 23.Ichikawa S; Guigonis V; Imel EA; Courouble M; Heissat S; Henley JD; Sorenson AH; Petit B; Lienhardt A; Econs MJ, Novel GALNT3 mutations causing hyperostosis-hyperphosphatemia syndrome result in low intact fibroblast growth factor 23 concentrations. J. Clin. Endocrinol. Metab 2007, 92 (5), 1943–1947. [DOI] [PubMed] [Google Scholar]
- 24.Ju TZ; Cummings RD, Protein glycosylation - Chaperone mutation in Tn syndrome. Nature 2005, 437 (7063), 1252–1252. [DOI] [PubMed] [Google Scholar]
- 25.Hiki Y; Kokubo T; Iwase H; Masaki Y; Sano T; Tanaka A; Toma K; Hotta K; Kobayashi Y, Underglycosylation of IgA1 hinge plays a certain role for its glomerular deposition in IgA nephropathy. J. Am. Soc. Nephrol 1999, 10 (4), 760–769. [DOI] [PubMed] [Google Scholar]
- 26.Moulder R; Bhosale SD; Goodlett DR; Lahesmaa R, Analysis of the plasma proteome using iTRAQ and TMT-based Isobaric labeling. Mass Spectrom. Rev 2018, 37 (5), 583–606. [DOI] [PubMed] [Google Scholar]
- 27.Greer T; Hao L; Nechyporenko A; Lee S; Vezina CM; Ricke WA; Marker PC; Bjorling DE; Bushman W; Li LJ, Custom 4-Plex DiLeu Isobaric Labels Enable Relative Quantification of Urinary Proteins in Men with Lower Urinary Tract Symptoms (LUTS). Plos One 2015, 10 (8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feng Y; Chen BM; Yu QY; Zhong XF; Frost DC; Ikonomidou C; Li LJ, Isobaric Multiplex Labeling Reagents for Carbonyl-Containing Compound (SUGAR) Tags: A Probe for Quantitative Glycomic Analysis. Anal. Chem 2019, 91 (4), 3141–3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kozak RP; Royle L; Gardner RA; Fernandes DL; Wuhrer M, Suppression of peeling during the release of O-glycans by hydrazinolysis. Anal. Biochem 2012, 423 (1), 119–128. [DOI] [PubMed] [Google Scholar]
- 30.Xu GG; Amicucci MJ; Cheng Z; Galermo AG; Lebrilla CB, Revisiting monosaccharide analysis - quantitation of a comprehensive set of monosaccharides using dynamic multiple reaction monitoring. Analyst 2018, 143 (1), 200–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miura Y; Kato K; Takegawa Y; Kurogochi M; Furukawa J; Shinohara Y; Nagahori N; Amano M; Hinou H; Nishimura SI, Glycoblotting-Assisted O-Glycomics: Ammonium Carbamate Allows for Highly Efficient O-Glycan Release from Glycoproteins. Anal. Chem 2010, 82 (24), 10021–10029. [DOI] [PubMed] [Google Scholar]
- 32.Yamada K; Hirabayashi J; Kakehi K, Analysis of O-Glycans as 9-Fluorenylmethyl Derivatives and Its Application to the Studies on Glycan Array. Anal. Chem 2013, 85 (6), 3325–3333. [DOI] [PubMed] [Google Scholar]
- 33.Gizaw ST; Gaunitz S; Novotny MV, Highly Sensitive O-Glycan Profiling for Human Serum Proteins Reveals Gender-Dependent Changes in Colorectal Cancer Patients. Anal. Chem 2019, 91 (9), 6180–6189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Williams TI; Saggese DA; Muddiman DC, Studying O-linked protein glycosylations in human plasma. J. Proteome Res 2008, 7 (6), 2562–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


