Figure 3.
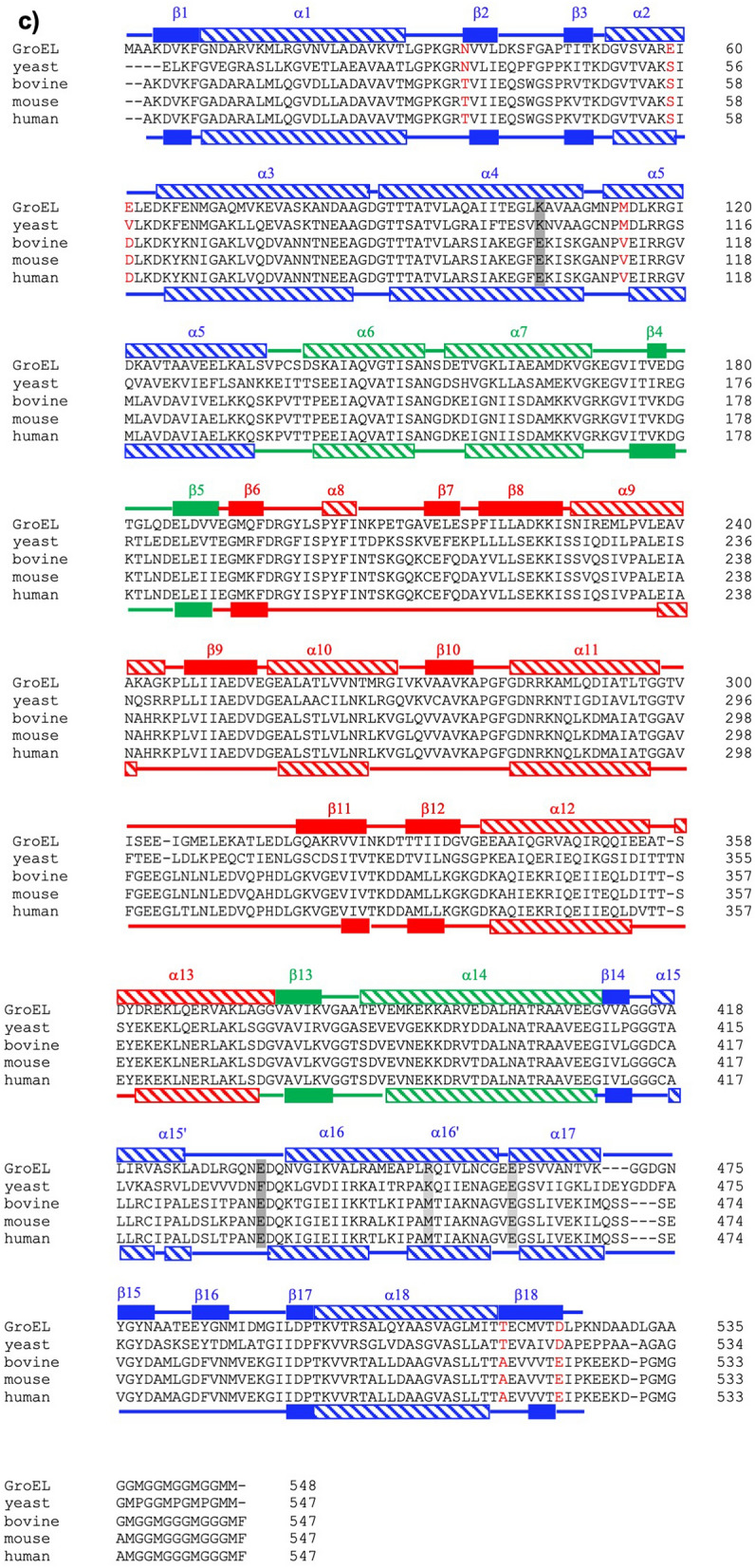
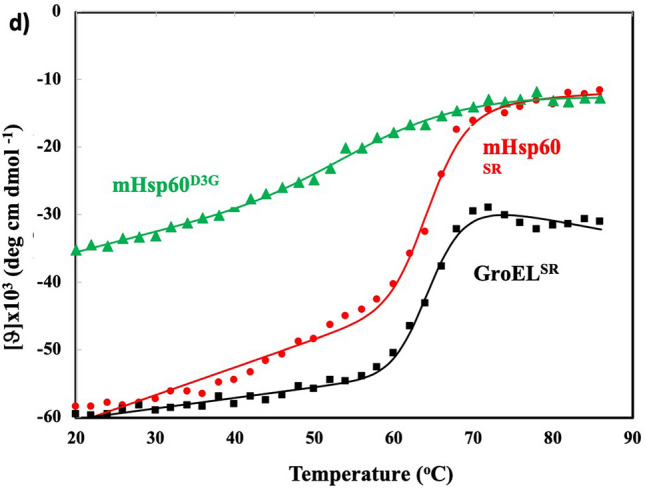
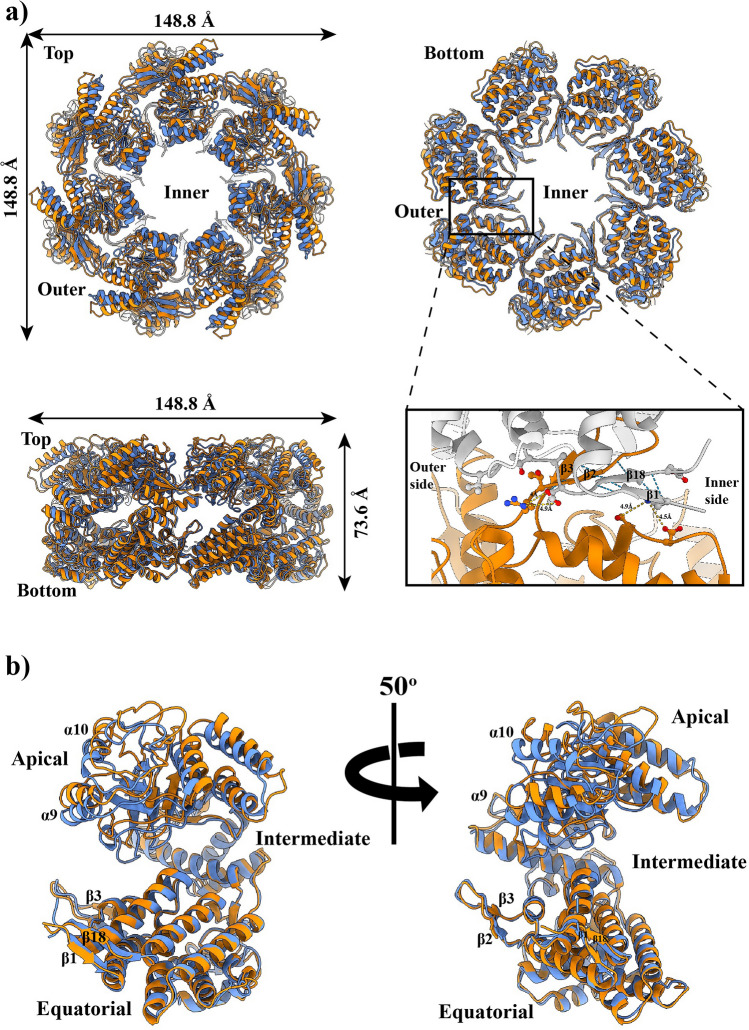
An expanded mHsp60 heptameric ring. (a) Superimpose of mHsp60 (orange) and a GroEL ring (blue; PDB: 5w0s) in top, bottom and side views. A four-stranded β-sheet, consisting of two strands β1 and β18 from one subunit (grey) and two strands β2 and β3 from the other subunit (orange), is located at the center of the cross-ring inter-subunit interface. Residues involved in inter-subunit interactions are highlighted in Fig. 4, and the atomic distances are summarized in Table 3. The mHsp60 heptamer and one GroEL heptamer were superimposed. (b) Overlay of a mHsp60 subunit with a GroEL subunit. The two equatorial domains were superimposed. (c) Sequence alignment among chaperonins from various sources. Secondary structures of mHsp60 and GroEL are based on Chimera: stripped rectangles for α helices and solid rectangles for β strands. Color scheme is the same as Fig. 2d. Residues involved in the inter-subunit interactions that are unique to human or higher eukaryote mouse and bovine (although yeast also has Ser at the corresponding S57 in human) are in red. Four residues forming the conserved inter-ring salt bridges are in dark (K105-E435) and light (R453-E462) shades, in GroEL numbering. For convenience, numbering of GroEL secondary structures is used here. (d) Thermal denaturation of single-ring chaperonins. The molar ellipticity (ϑ) was measured at 222 nm. The solid lines are non-linear regression analysis (Eq. 1). The derived ΔHvh, ΔGunfolding and Tm values are listed in Table 4.