Abstract
The Integrated Stress Response (ISR) is a powerful and conserved signaling pathway that allows for cells to respond to a diverse array of both intracellular and extracellular stressors. The pathway is classically responsible for coordination of the cellular response to amino acid starvation, ultraviolet light, heme dysregulation, viral infection, and unfolded protein. Under normal circumstances it is considered pro-survival and a necessary mechanism through which protein translation is controlled. However, in cases of severe or prolonged stress the pathway can promote apoptosis, and loss of normal cellular phenotype. The activation of this pathway culminates in the global inhibition of cap-dependent protein translation and the canonical expression of the activating transcription factor 4 (ATF4). The eye is uniquely exposed to these stressors due to its environmental exposure and relative isolation from the circulatory system which are necessary for its function. In this review we will summarize the growing body of evidence of the role of the ISR in the context of ophthalmology, with special interest on the cornea and anterior segment. We will discuss how this pathway is critical for the proper function of the tissue, its role in development, as well as how targeting of the pathway could alleviate key aspects of diverse ophthalmic diseases.
Keywords: ATF4, ISR, ISRIB
Introduction:
The Integrated Stress Response (ISR) is a critical pathway that is required for cellular response to external and internal cellular stressors1. Principally, it provides a mechanism by which a cell can alter its protein production dynamics and shut down global cap-dependent protein synthesis.2 This functions as a brake on protein synthesis allowing the cell time to adapt to the new environment by slowing the rate of protein synthesis and therefore decreasing demand for energy3 and amino acids.4 Simultaneously, the cell can increase the production of proteins involved with cell survival,5 amino acid synthesis,6 and autophagy7 through the key transcription factor of the ISR, activating transcription factor-4 (ATF4). The activation of the ISR is canonically carried out in response to signaling from four kinases that are principally responsive to; amino acid and glucose deprivation (general control non-depressible protein 2, GCN2), 4, 5 heme deficiency (heme-regulated inhibitor, HRI),8 viral infection (double-stranded RNA-dependent protein kinase, PKR),9 hypoxia10 and the accumulation of unfolded proteins in the endoplasmic reticulum (PKR like endoplasmic reticulum kinase, PERK).11 Activation of the ISR results in a reduction of global protein synthesis and an induction of specific genes that aid in cellular recovery.1, 2 A short-lived ISR is a pro-survival response aimed at relieving stresses and regaining protein homeostasis, while a sustained ISR can result in cell death.12 Therefore, it is commonly believed that the ISR is a double-edged sword whose actions have been correlated with a diverse field of human diseases including neurodegeneration,13 endocrine disorders,14 cancer,15, 16 autoimmune disorders,17 and infections.18
Unlike other tissues, the eye is uniquely exposed to all the potential stressors that can activate the ISR being both exposed to the environment, and being relatively isolated form the circulatory system19, 20. The response to these stressors in the eye is also limited, neovascularization in the eye has been associated with deleterious diseases and loss of vision.21, 22 Viral infection of the eye is also pernicious with the eye being a privileged site that does not, and indeed cannot, utilize the full extent of the immune system to clear infection.23, 24 The activation and consequences of an active ISR have often been observed, or eluded to, in diverse ocular morbidities affecting all tissues of the eye. Reports of ISR involvement have been published in, macular degeneration,21 neurodegeneration,25 diabetic retinopathy,26 cataracts,27 dry eye disease,28 and keratoconus.29 In animal models, the ablation of ATF4 and other components of the pathway leads to profound ocular phenotypes in development which leads us to suggest that ISR and ATF4 are key modulators of ophthalmic diseases.30–32 Critically, the ISR is considered a druggable target, with several compounds that target the pathway under investigation for diverse pathologies.33–35 In this review, we update the pathophysiologic roles of the ISR in ocular diseases, with special interests in disorders of the anterior segment. We further explore the potential for small molecules in modulating key components of the ISR to modulate their activity, and finally discuss the available animal models of ISR disruption, their ocular phenotypes, and their usefulness in studying the role of the ISR in the eye.
Integrated Stress Response (ISR)
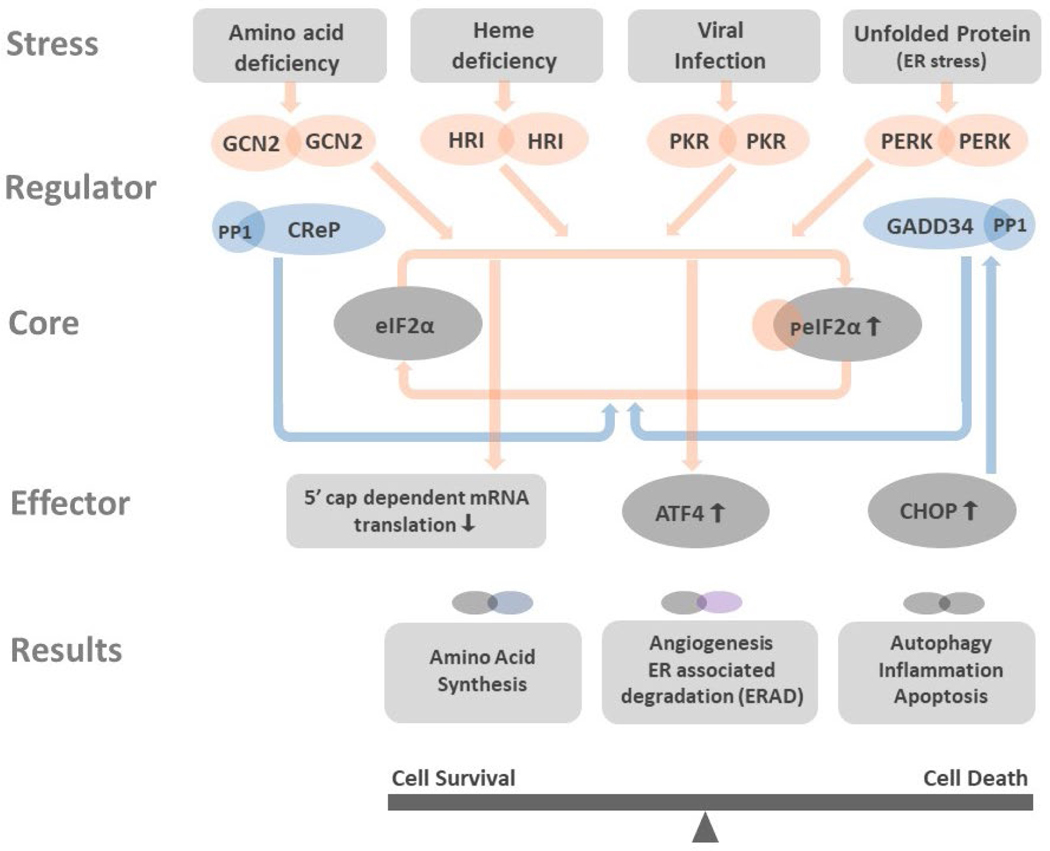
The ISR is the central node by which the cell coordinates the dynamics of protein production in response to environmental and intracellular stressors.1, 2 The ability to decrease the rate of protein production and alter phenotype in response to acute and chronic stress situations is critical for survival of the cell, and the whole organism.36 In humans, the ISR sensors comprise four eIF2α kinases:37 GCN2,38 HRI,8 PKR,39, 40 and PERK.11, 41 These kinases respond to diverse stresses: GCN2 to amino acid and glucose deprivation,42 HRI to iron deficiency,43 PKR to viral infection,9 and PERK to protein misfolding in the ER, the so-called ER stress.44 Upon activation of its respective stimulus each kinase dimerizes, auto-phosphorylates, then phosphorylates Ser51 of eIF2α as demonstrated 37in Figure 1.
Figure 1: Integrated stress response affects cell functioning.
Amino acid deficiency, heme deficiency, viral infection, and unfolded protein stress in the endoplasmic reticulum (ER stress) activate GCN2, HRI, PKR, and PERK. All these kinases phosphorylate eIF2α, the core member of ISR. Phosphorylated eIF2α (peIF2α) impedes the 5’cap-dependent mRNA translation (Figure 2 in details) and results in global reduction of protein synthesis. However, few genes as ATF4 and CHOP have alternative translation machinery and are less influenced by eIF2 dysfunction. The preferentially translated ATF4 is the key effector in ISR. ATF4 couples with another ATF4 or its interacting partners to form homo- and heterodimers that bind to DNA targets and control the expression of genes that participated in amino acid synthesis, angiogenesis, ERAD, autophagy, inflammation, and apoptosis. Depends on the disease context and the duration of ISR, the downstream processes of ISR can aid in cell survival or bring cell death. CHOP is one key factor that can be promoted by ATF4. CHOP not only activates autophagy, inflammation, and apoptosis, but also induces the expression of GADD34, a phosphatase coupled with PP1 to dephosphorylate peIF2α. The dephosphorylation of peIF2α terminates ISR and resumes protein synthesis. At non-stressed cell, the CreP-PP1 complex phosphatase constantly operates for maintaining low level of peIF2α and protein homeostasis.
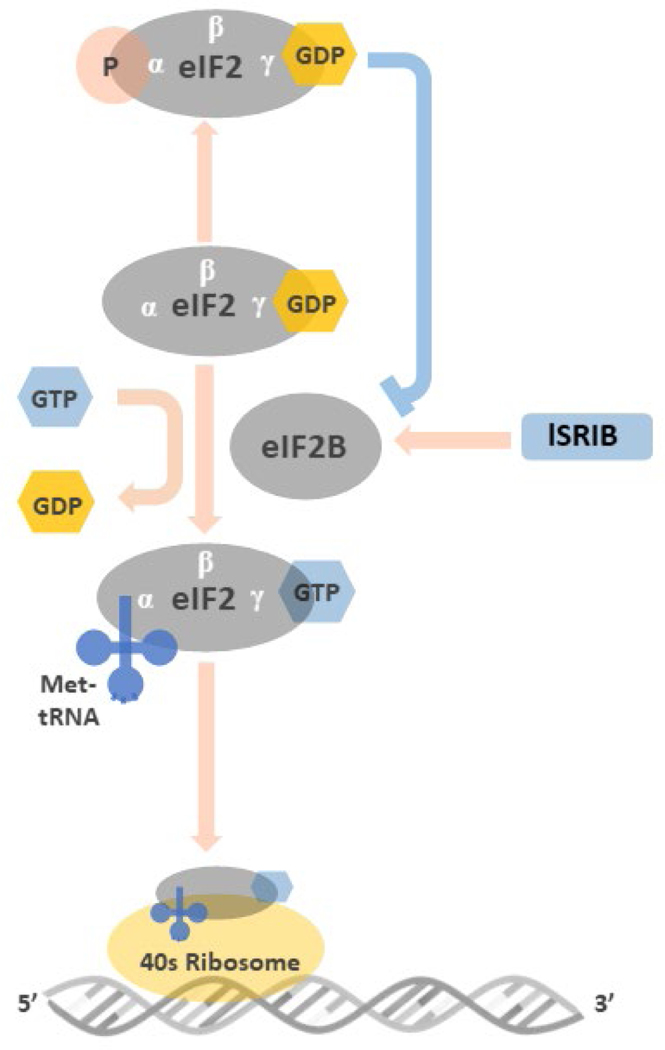
The core mediator of the ISR response is the eIF2α subunit, being a key element in the eukaryotic initiation factor 2 complex (eIF2, consisting of α, β, and γ subunits),1, 37 this is a central subunit required for the initiation of mRNA translation. Under normal conditions, the eIF2 complex binds a GTP, and a met-tRNA molecule and presents these to the 40S ribosomal subunit.45 This assembly is a key rate limiting step in the initiation of mRNA translation,46, 47 therefore the function of the ISR is to regulate the availability of eIF2α by decreasing the pool of the subunit available to initiate ribosome assembly1, 2 (Figure 2).
Figure 2: Illustration of the roles of eIF2 and peIF2 in the initiation of mRNA translation.
Before binding to met-tRNA, the GDP bound to the γ subunit of eIF2 must be switched to GTP. This GDP-GTP exchange process is mediated by eIF2B. The met-tRNA-GTP-eIF2 ternary complex then delivers the met-tRNA to 40S ribosome, a key step for the initiation of mRNA translation. At the stressed condition, once the ISR is activated and the GDP-eIF2 is phosphorylated in its α subunit, the structural change makes GDP-peIF2 no longer a suitable substrate of eIF2B but transforms to an inhibitor of eIF2B. The inhibition of eIF2B by GDP-peIF2 further reduces the pool of functional GTP-eIF2 to bind met-tRNA, and lowers the global protein synthesis via 5’cap-dependent mRNA translation. ISRIB rescues protein translation by stabilization and increasing eIF2B abundance that counteracts low level of GDP-peIF2α in treated cells.
Under cellular stress, any of the above-mentioned kinases can phosphorylate Ser51 of eIF2α (peIF2α). This event causes the subunit to increase its affinity for the guanine nucleotide exchange factor, eIF2B.46 Once bound peIF2α inhibits the ability of the eIF2B to exchange GDP for GTP resulting in a decrease in the available pool of free GTP-eIF2 and decreasing the rate of translational initiation33. The amount of eIF2B in the cell is significantly less than eIF2α so only a small increase in peIF2α can sequestrate all the available eIF2B and effectively shut down cap dependent mRNA translation.48, 49 This reduction of global protein synthesis via the 5’ cap-dependent mRNA translation serves a number of cytoprotective roles.37 In conditions of amino acid shortage50 or iron deficiency,51 it reduces the rate at which these nutrients are consumed, during viral infection it slows viral replication by impeding viral protein synthesis52, 53. While in ER stress, its activation decreases the rate of proteins entering the ER, thereby relieving the overburdened organelle.54
However, certain mRNAs that encode for proteins participating in cell responses to cellular stress are not affected or are indeed enhanced in the presence of eIF2α phosphorylation. Examples of these mRNAs include the ATF4,55 C/EBP homologous protein (CHOP),56 and growth arrest and DNA-damage 34 (GADD34).57 This subset of mRNAs do not require 5’ cap recognition during translation, instead utilizing a re-initiation mechanism based on the direct recruitment of ribosomes to internal ribosome entry sites.55–57 As Figure 1 demonstrates, ATF4 being the best characterized effector of the ISR, is a transcription factor which has several dimerization partners that collaborate in the regulation of gene transcription and direct cellular outcomes36 in amino acid transportation,58 oxidative stress,50 glucose metabolism,59 autophagy,60 angiogenesis61 and protein homeostasis.62
Timely termination of the ISR allows protein synthesis to recover once the ISR stimuli is relieved. As Figure 1 shows, at low stressed conditions, the constitutive repressor of eIF2α -protein phosphatase 1 (CReP-PP1) complex63 sustains a low level of peIF2α by removing phosphate group and restoring translational homeostasis. However, during chronic stress, ATF4 mediates activation of CHOP, which cooperates with ATF4 to induce growth arrest and GADD34 expression. GADD34 then couples with PP1 to terminate the ISR by dephosphorylation of peIF2α.64 This feedback mechanism allows for protein synthesis to continue and contributes to increased ER stress and induction of cell apoptosis.65 Therefore, the downstream execution of ATF4 can be cell apoptosis,66 cell-cycle arrest,67 and senescence.68
In brief, acute activation of the ISR leads to temporary shutdown of the universal protein synthesis, whilst simultaneously activating pro-survival genes through ATF4 activation. However, in conditions such as chronic stress, prolonged activation of the ISR may activate CHOP or other pro-apoptotic genes and lead to cell death.2
Pathophysiological association of ISR and Ocular Diseases
As Figure 1 summarizes, ISR activation by different stresses converge in the phosphorylation of eIF2α and the increase of ATF4. Therefore, alongside with the expression of ISR regulators (kinases), peIF2α and ATF4 are the common indicators of ISR activity in ocular disorders2. In addition, CHOP expression frequently suggests the pro-apoptotic state or cell death as the result of ISR activation in disease models.65
(1). Cornea and Conjunctiva
Fuchs endothelial corneal dystrophy, a disorder of the corneal endothelium that is characterized by loss of endothelial cells and abnormalities of Descemet’s membrane, may result in progressive corneal edema.69, 70 Enlargement of rough ER of endothelial cells has been demonstrated in Fuchs dystrophy specimens. Meanwhile, significantly higher peIF2α and CHOP expression were quantified in Fuchs dystrophy corneal endothelium as compare to the non-Fuchs dystrophy controls.71 Okumura et al also reported elevated PERK activation and CHOP expression in cultivated human Fuchs endothelial cells as compared to its normal controls.72 Both studies have shown that accumulation of unfolded proteins may induce corneal endothelial cell apoptosis.
Keratoconus, a multifactorial disease that is characterized by progressive thinning and weakening of the cornea, could lead to severe visual impairment in young adults.73 Mass spectrometric analysis of keratoconic corneal proteins has suggested increased ER stress, oxidative stress, and apoptosis in the keratoconic stromal proteome.74 We have reported elevated peIF2α and ATF4 in cultivated stromal keratocytes and corneal buttons from keratoconus patients as compared to those from normal controls.29 Our findings indicate abnormal ISR activity may participate in keratoconus pathogenesis.
Another corneal disease known to be related to abnormal ISR is granular corneal dystrophy type 2 (GCD2), which is caused by the mutation in the transforming growth factor β-induced (TGFBI) gene. Diseased GCD2 corneal fibroblasts have been shown to accumulate mutant TGFBI-encoded protein (TGFBIp).75 Choi et al has also demonstrated a higher level of PERK activities in GCD2 corneal fibroblasts, and GCD2 fibroblasts were more susceptible to ER-stress induced cell death than were the normal controls.76
Herpes simplex virus type 1 (HSV-1) is an alphaherpesvirus that is recognized as the most common cause of corneal blindness in developed countries.77 Ocular involvement can present as primary infection or recurrence from latent disease.78 HSV-1 is known to be able to disarm the ISR of host cells in their early stage of infection.79 Us11, a HSV-1 protein, can bind dsRNA and block PKR activation.80 HSV-1 also expresses an ortholog of GADD34 called γ34.5, that results in eIF2α dephosphorylation to ensure protein synthesis and offset the activation of PKR and PERK during viral infection.81 By maintaining a pool of unphosphorylated eIF2α, HSV-1 avoids antiviral cellular translational arrest, meanwhile, it prevents potentially hurtful downstream ISR transcription from ATF4 and CHOP, and the induction of autophagy.24 The ability of countering antiviral response of infected cells promotes HSV-1 neuroinvasion and periocular replication following corneal infection.82
ER stress was also known to involve in the pathogenesis of chronic inflammatory and autoimmune diseases, including dry eye. Pflugfelder’s group has demonstrated interferon-γ (IFN-γ) could induce unfolded protein response and the expression of ATF4, and thus resulted decreased mucin synthesis and reduced goblet cell proliferation in the cultivated conjunctival goblet cell harvested from C57BL mice. Interestingly, the conjunctival goblet cells cultivated from IFN-γ knockout or IFN-γ receptor knockout mice had increased proliferation as compared to that from the wild type. In other words, inflammatory related ER stress participates in the mucin-deficiency dry eye via modulating the mucin production and goblet cell proliferation.
(2). Lens
Lens epithelial cells coordinate the development of the entire ocular lens. During cellular maturation, most lens fiber cells lose their nuclei and mitochondria.83 However, the anterior epithelial cells remain mitotically active as a stem cell niche producing secondary fiber cells.84 The ISR is highly associated with lens development and degeneration as the absence of secondary lens fibers and severe microphthalmia30 have been shown in ATF4 deficient mice. In addition, interruption of lens autophagy could lead to the loss of lens differentiation and the failure of lens resistance to stresses, resulting in cataract formation.85, 86 Cataract, which has long been the leading cause of blindness globally,87 can be induced by senility and physical and chemical stresses.88 A large variety of ER stressors were found to induce the production of reactive oxygen species in cultivated lens epithelium with or without the induction of cell apoptosis.89 Increased level of ATF4 and CHOP were documented in human aged lenses.89 The activation of ER stress pathways were also reported in high-myopia related cataract90 and congenital cataract.27 Accordingly, ATF4 related pathways is key for the evolution of normal lenses. The ER stress and its consequences may be one of the initiation factors of many types of cataracts.27, 91
(3). Retina and Glaucoma
Accumulation of unfolded protein activates the PERK pathway and its related pro-apoptotic circuits, which are implicated in the pathogenesis of many diseases involving different layers of the retina and optic nerve.92 Diabetic retinopathy (DR), a leading cause of visual loss in the working-age population, has long been recognized as a microvascular disease.93 The pathologies in DR include pericyte loss and vascular endothelial cell apoptosis in responses to hyperglycemia.94 Zhang et al have proved intermittent high glucose can induce ER stress in human retinal pericytes, and is related to the inflammatory responses in DR mouse models.26, 95 Elevated ATF4 was further demonstrated in the aqueous humor and vitreous of proliferative diabetic retinopathy patients by Wang et al. In contrast to DR, age-related macular degeneration (AMD) is the top cause of blindness among the aged group in the developed world.96 AMD is characterized by dysfunction of retinal pigment epithelium (RPE) cells and their over expression of vascular endothelial growth factor (VEGF).21, 97 Roybal et al reported oxidative stress activates the secretion of VEGF in human RPE cells by an ATF4 dependent mechanism. The ATF4 complex binds to the VEGF gene and transactivates its expression.98 Therefore, activation of the ISR under oxidative stress may be one of the contributing factors to neovascularization in retinal diseases.99 Alongside DR and AMD, another key contributor to global visual impairment is glaucoma, a group of optic neuropathies characterized by progressive loss of retinal ganglion cells.100 ER stress was documented to play a major role in the pathogenesis of myocilin mutation associated glaucoma and glucocorticoid-induced ocular hypertension in mouse models.25, 101 Trabecular meshwork tissue lysate form glaucoma patients also demonstrated increased ER stress markers including ATF4 and CHOP as compared to those age-matched normal controls.102
In summary, the ISR aids in cellular adaptation and defense in the eye. Inactivation of the ISR by viral proteins allows viral replication and tissue destruction. Moreover, the eye is consistently under oxidative and metabolic stresses, chronic stimulation of the ISR may lead to cell death, and ISR stimulation is observed in both anterior and posterior segment ocular disorders.
Modulators of ISR and their possible therapeutic applications
Many toxins or drugs induce extensive ISR,103 including arsenic,104 tunicamycin,105 thapsigargin,106 and mitomycin C.107 Environmental arsenic contamination in drinking water has been a global health issue that was associated with many cancers.108 Arsenic generates oxidative stress and mediates ER and mitochondrial cross-talks that results in apoptosis via ATF4 regulated pathways.104 Arsenic induced-VEGF production in retinal pigmented epithelium via eIF2α-ATF4 branch has suggested non-hypoxic stresses also contributed to VEGF expression.98 Tunicamycin, an antibiotic mixture produced by Streptomyces lysosuperificus, has antimicrobial activity against bacteria, fungi, and viruses. Tunicamycin not only impairs protein glycosylation, but also depletes the calcium in ER which further aggravates unfolded proteins stress.105 Tunicamycin has not been used as human medicine103 due to its toxicity, but has been broadly applied as an ER stressor in studying various pathological and physiological processes of diabetes and asthma.109, 110 Thapsigargin is a highly potent drug isolated from the plant Thapsia garganica L (Linnaeus),106 its cytotoxicity is derived from its ability to inhibits calcium transport leading to calcium depletion in ER. Therefore, in addition to ER stress-related cell death, thapsigargin induces concomitant increase in free cytosolic calcium is also a potent pro apoptotic signal in cells.106 Mitomycin C, is a reactive oxygen species (ROS)-generating anticancer drug. Mitomycin C can induce human fibroblast apoptosis via PERK pathway,107 and its anti-fibrotic effect has been applied in many ophthalmic surgeries such as pterygium excision111 and glaucoma filtering surgery.112
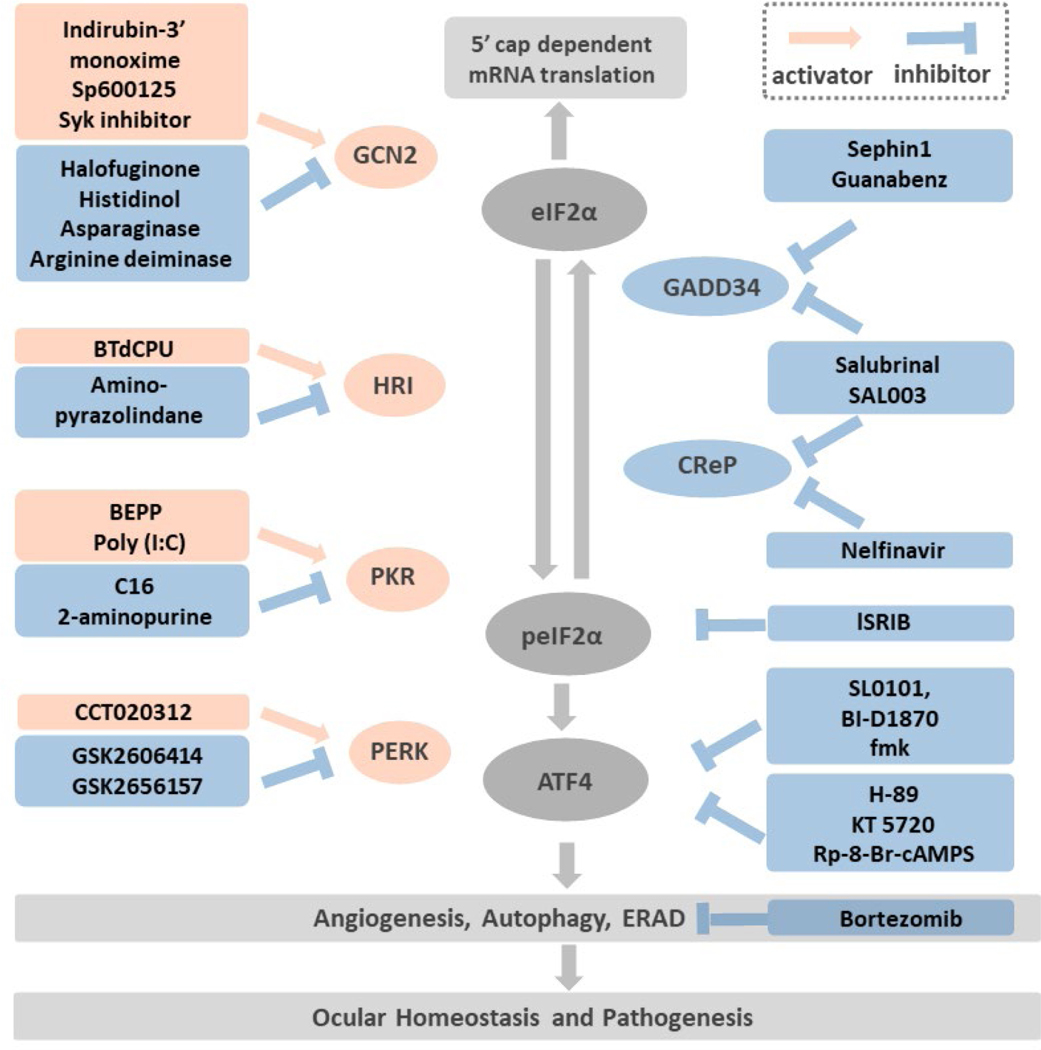
Other than these general ISR inducers, there are several approaches for therapeutic activation or inhibition of the ISR by modulation of specific targets: (1) counteracting the effect of peIF2α; (2) activation or inhibition of the eIF2α kinases; (3) inhibition of the eIF2α phosphatase; (4) post-translational modification of ATF4; (5) altering the ATF4 downstream pathways. Here we elaborate the most studied small molecules targeting ISR pathways, especially the highly potent ISR inhibitor, ISRIB. We also summarize the molecules that have been applied in the ophthalmic field (Figure 3). Detailed review of small molecules modulating the ISR network are listed.2, 33–35, 113
Figure 3: Targeting the druggable ISR pathways.
Modulation of ISR can be achieved by: (1) Targeting the eIF2α kinases: the GCN2 activators include Indirubin-3’ monoxime, Sp600125, Sky inhibitor; the GCN2 inhibitors consist of halofuginone, histidinol, asparaginase, arginine deiminase. The HRI activator BtdCPU and the inhibitor, amino-pyrazolindine. The PKR activators are BEPP and Poly (I:C). The PKR inhibitors are C16 and 2-aminopurine. The selective PERK activator is CCT020312 and the inhibitors are GSK2606414 and its more selective derivative GSK2656157. (2) Targeting the peIF2α phosphatase: Salubrinal and SAL003 inhibit both the inducible phosphatase GADD34 and the constitutive expressed CReP. In addition, Sephin 1 and Guanabenz inactivate GADD34, while Nelfinavir inactivates CReP. (3) Antagonist to the peIF2α: as Figure 2 shows, peIF2α inhibits the eIF2B. ISRIB is hypothesized to stabilize eIF2B and offsets the peIF2α effects while the peIF2α concentration is low. (4) Inhibition of ATF4 post-translational phosphorylation: RSK2 and PKA are two kinases that phosphorylate ATF4 and increase its activity. The RSK2 inhibitors, SL0101, BI-D1870, fmk and the PKA inhibitors, H-89, KT 5720, Rp-8-Br-cAMPS are potential compounds that inhibit ATF4 phosphorylation. (5) Targeting pathways downstream of ATF4: among the cellular functions regulated by ATF4, angiogenesis, autophagy and ERAD played important roles in ocular homeostasis and pathogenesis such as dry eye, diabetic retinopathy, age-related macular degeneration. Bortezomib, a proteasome inhibitor impedes ERAD has been demonstrated with neuro-protective and anti-inflammatory effects in vivo.
(1). Counteract the effect of peIF2α: eIF2B activator
As Figure 2 illustrated, eIF2B catalyzes the GDP-GTP exchange on eIF2. While the eIF2α is phosphorylated, it transforms from a substrate into potent inhibitor of eIF2B and reduces its catalyzing ability.1, 46 A small molecule ISR inhibitor, ISRIB, discovered by Walter’s group, binds in the pocket formed at the junction of eIF2B β- and δ-subunits, driving the subunits to fully assemble the eIF2B decamer complex with GDP exchange ability.114–116 ISRIB, therefore, rescues protein translation by stabilizing and increasing eIF2B complex abundance and counteracting low levels of peIF2α in treated cells.116, 117 However, when the concentration of peIF2 exceeds a certain threshold-that is, when the ISR is strongly activated, more eIF2B are trapped by peIF2, and ISRIB can no longer restore the drained pool of eIF2B complex. Thus, ISRIB does not abolish the ISR’s cytoprotective effects in cells in which the ISR is strongly activated.1 ISRIB has good potency (nM) and brain penetrance, and optimization of ISRIB has led to the discovery of analogs with picomolar activity.117, 118 In vitro, ISRIB treatment partially restores the cellular protein translation rates in cultivated neuronal model of amyotrophic lateral sclerosis.119 In vivo, ISRIB partially recovers the cellular protein translation rates in prion-infected mice.120 ISRIB also reverses some aspects of cognitive deficit in traumatic brain injury mice121 and Down syndrome mice.122 In the ophthalmic field, our group was the first to report that activation of the ISR can recapitulate the keratoconus phenotype in normal corneal keratocytes in vitro. Conversely, by blocking the ISR with ISRIB, the production of matrix metalloprotease (MMP)-9 is reduced and the synthesis of collagen in keratoconic fibroblasts increases in vitro, relieving many of the hallmarks of keratoconus.123 Therefore, targeting of the ISR through small molecules may be a promising therapeutic path for ophthalmic disease.
(2). Target the eIF2α kinases
Activators of eIF2α kinases
Pharmacological activation of ISR signaling can be achieved by activating eIF2α kinases. Halofuginone,124 histidinol,116 asparaginase,125 and arginine deiminase126 are known to be GCN2 activators; BTdCPU activates HRI;127 BEPP and poly (I:C) are PKR activators;128, 129 and CCT020312130, 131 is a selective PERK activator. These potential drugs were mostly studied for cancer treatment, and the application in ophthalmology fields is just emerging.33, 35 In vivo, oral administrated or intraperitoneal injected halofuginone has shown potent inhibitory effects on angiogenesis progression in a mouse corneal neovascularization model.132 Arginine deiminase catalyzes the deimination of proteins, a nonreversible post-translational conversion of protein-bound arginines to protein-bound citrullines. Elevated levels of protein deimination are reported in human neurological diseases,133 the retina tissue of AMD patients,134 and the optic nerve of open-angle glaucoma eyes.135 These findings suggest GCN2 may be one of the treatment targets of AMD and glaucoma. Poly (I:C), polyinosinic:poycytidylic acid, has similar structure to the double-stranded RNA which leads to PKR activation.129 In vivo, topical and systemic administrated Poly (I:C) has been known for decades to induce tear interferon against HSV in rabbit and human eyes.136, 137
Inhibitors of eIF2α kinases
Pharmacological inhibition of the ISR can be achieved by inhibition of eIF2α kinases. Three structurally similar compounds indirubin-3’-monoxamie, SP600125 and a SyK inhibitor inactivate GCN2;138 amino-pyrazolindine inhibits HRI;139 C16 and 2-aminopurine are frequently used for inhibition of PKR;140, 141 and GSK260614 and its analogue GSK2656157 inactivate PERK.142, 143 However, the application of eIF2α kinase inhibitors in ophthalmic researches is scarce. In vitro, GCN2 inhibitors, Indirubin-3’-monoxamie, SP600125, and SyK inhibitor decrease the phosphorylation of eIF2α in mouse embryonic fibroblast cells after UV irradiation.138 Yet, these compounds are poorly specific for GCN2, and additional studies on structure-activity relationship are essential to increase their specificity and potency in treating eye diseases. In vitro, Jiang et al has reported GSK2606414, the highly selective PERK inhibitor, suppressed RPE cell proliferation in a dose-dependent manner. Meanwhile, GSK2606414 treatment reduced the level of peIF2α, CHOP, and VEGF mRNA expression in RPE cells under ER stress.144
(3). target eIF2α phosphatase
Dephosphorylation of eIF2α is the key step of ISR termination and restoration of protein synthesis for normal cellular functions. As shown in Figure 1 and 3, in mammals, two phosphatases are responsible for the dephosphorylation of peIF2α, the CReP63 and GADD34.145 As the name suggested, CReP normally operates in unstressed cells for maintaining low level of peIF2α. While GADD34 expression is induced by ATF4 and CHOP, and acts as a negative feedback loop to resume protein synthesis once the stress is relieved.64 Salubrinal and its analogue SAL003, are inhibitors of both GADD34 and CReP.123, 146 Guanabenz and its derivative, Sephin 1 are known to inhibit GADD34.146 Nelfinavir, a HIV protease inhibitor downregulates CReP and increases peIF2α.147 Among these peIF2α phosphatase inhibitors, salubrinal has been shown to result in high levels of peIF2α. It has also been demonstrated that salubrinal blocked the replication of HSV in cell culture models.148, 149 In vitro, Salubrinal treatment protects trabecular meshwork cells against ER stress150 and RPE cells against toxins.151, 152 However, we found SAL003 stimulates the ISR and leads to apoptosis of normal fibroblasts cultivated from donor corneas.123
(4). Post-translational modification of ATF4
There are no reported molecules that can directly bind and inhibit ATF4. Transcription factors are largely poor drug targets due to the inefficiency of small molecules to halt protein-DNA and protein-protein binding interfaces.153 However, ATF4 function is dependent on extensive post-translational modifications, particularly phosphorylation, that modulates its transcriptional activity and degradation. Ribosomal S6 kinase (RSK2) and protein kinase A (PKA) are two kinases that phosphorylate ATF4 and enhance its transcriptional activity.154, 155 Reversible RSK2 inhibitors SL0101 and BI-D1870, and the irreversible inhibitor fmk have been developed.156–158 PKA inhibitors H-89 and KT 5720 are also available yet with low selectivity.159 Recently, another PKA antagonist Rp-8-Br-cAMPS with better selectivity has been developed.160 However, RSK2 and PKA both work on multiple protein responses for different cellular functions,161, 162 and the use of these compounds as ATF4 inhibitors will need careful validation to support that the observed effects can be contributed to ATF4 inhibition. As yet, the application of ATF4 inhibitor in treating eye disease has not been reported.
(5). Target pathways downstream of ATF4
As Figure 1 and 3 demonstrate, ATF4 transcriptionally regulates genes involved in different cell functioning.33 Disruption of the pathways downstream of ATF4 is also a valid approach to interfere the effects of ISR. Insufficient vascular supply results in hypoxia, nutrient deprivation, lower ATP production and protein misfolding. These factors lead to ATF4 expression in ischemic tissue,163 and drive the expression of VEGF22 and pro-angiogenesis cytokine, interleukin (IL)-8.164 Targeting VEGF or pro-angiogenesis interleukins has long been the promising treatment for ocular vascular neogenesis disorders.165 Another potential therapeutic target is autophagy, the catabolic process responsible for lysosome-dependent degradation and recycling.166 The expression of ATF4 and CHOP upregulate autophagy genes,167 and dysregulated autophagy disturbs cellular homeostasis and has been linked to many ocular disorders, including dry eye, corneal dystrophy, keratoconus, cataract, glaucoma, and AMD.85, 168 The other key mechanism for maintaining cellular homeostasis is the ER associated protein degradation (ERAD), which through the accumulation of unfolded proteins activates the PERK-ATF4 pathway.169 Bortezomib is a specific inhibitor of the proteasome that impedes ERAD170 which has shown neuro-protective effects in retinal ischemia-reperfusion injury in rat171 and anti-inflammatory effects in experimental autoimmune uveitis (EAU) mice after intraperitoneal injection.171, 172
Animal models in ISR research
Investigations of the ISR is an emerging field based on its established role in assorted human pathologies2 and because the pathway is considered a “druggable target”. Nonetheless, most mechanistic studies have been implemented in cell culture systems exposed to the stressors. In Table 1, we summarize relevant studies in animal models that have revealed novel and specialized functions of the ISR in distinct organisms in vivo.173 The most profound of which is the ATF4 knockout mouse which presented with lens anomaly and microphthalmia.30
Table 1:
Consequence of the genetic manipulation of ISR components in mice
| Member | Model | Development | Phenotypes | Ref. |
|---|---|---|---|---|
| ATF4 | CREB−/− | Retarded | Defective lens development, and microphthalmia Anemia Dwarfisms and severe skeletal abnormalities |
30, 59, 174, 176, 177 |
| CREB+/− | Normal | No specific phenotype | ||
| CREB−/− and P53−/−double KO | -- | Normal eye morphology, lower number of lens fibers | ||
| GCN2 | GCN2−/− | Normal | Developmental delay occurred if being fed with leucine deficit diet | 178 |
| HRI | HRI−/− |
Normal | Macrocytosis and hyperchromia | 51 |
| PKR | Pkr−/− | Normal | No specific phenotype | 179 |
| PERK | Perk−/− | Retarded | Impaired insulin secretion and hyperglycemia; Growth retardation and abnormal bone development |
180–183 |
| Perk−/− | -- | Acute diabetes | 194 | |
| Perk loxP/loxP & CamkIIα-Cre | -- | Behavior change | 184 | |
| GADD34 | GADD34−/− | Normal | No signs of disease or abnormal phenotypes at least the first 12 months of life | 145 |
| CReP | Ppp1r15b−/− | Retarded | Half size, pale skin, impaired erythropoiesis, fail to survive in postnatal day 1 | 186 |
| Ppp1r15b+/− | Normal | No specific phenotype | ||
| EIF2α | Homozygous non-phosphorylatable KI (S51A) | Lethal | Severe hypoglycemia. Lethal within 24 hours of birth. |
188 |
| Heterozygous non-phosphorylatable KI (S51A) | Normal | Glucose intolerance, increased weight gain if been fed with high fat diet, pancreatic β-cell failure. | 189 | |
| Conditional β-cell specific KO | -- | Decreased insulin secretion, type 2 DM | 190 | |
| CHOP | CHOP−/− | Normal | Reduced performance in memory-related behaviors | 192 |
KO: knock out; KI: knock in
(1). ATF4 knock out mice
ATF4 is a member of the cAMP-responsive element-binding protein (CREB) family and has multiple roles in cell differentiation and organ morphogenesis.30, 174, 175 ATF4 heterozygous mice are viable, yet only 30% of ATF4 knockout (CREB−/−) mice survive to maturity.176 ATF4 deficiency leads to mutations in eye-lens development and hair-growth, accompanied by pancreatic hypoplasia skeletal defects, and growth retardation.30, 59, 174, 176, 177 Tanaka et al generated ATF4−/− mice and noted severe microphthalmia and lens degeneration due to cell apoptosis without the formation of lens secondary fiber cells. Rescue of the ATF4 mutant phenotype by lens-specific gene expression not only recovered its lens secondary fibers but also induced hyperplasia of these fibers.30 Hettmann et al have also demonstrated microphthalmia due to absence of lens on ATF4−/− mice.176 They noted the defect appeared to be specific for the lens, as no gross anomalies in the retina or cornea could be detected. Interestingly, the ATF4−/− and p53−/− double-homozygous mutant mice resulted in a marked suppression of microphthalmia, bringing together the DNA damage response, and the ISR in development. The lens developed in the double mutant model; however, it contained a lower number of fibers as compare to that of the wild type. Therefore, the authors proposed that there may be both p53-dependent and p53-independent effect of ATF4 deficiency participating in the differentiation and survival of lens fiber cells.176 Overall, these animal studies support the functions of ATF4 in modulating cell cycle, apoptosis, and metabolism in diverse tissues.
(2). EIF2α kinase gene knockout mice
In the 1990s, the physiologic functions of eIF2α kinases were elaborated. Kinase gene knockout mice were bred for development and phenotype observation51 as listed in Table 1. Interestingly, Gcn2−/− mice have no observable phenotype mutation in a non-stressed status but present with developmental delay while being fed with leucine deficit diet.178 HRI−/− mice are viable, fertile, and without gross abnormalities. Mild macrocytosis and hyperchromia were observed in the HRI−/− mice in the absence of stress that indicates the physiological adaptation of red blood cells to iron deficiency.51 Pkr−/− mice have normal development and similar response to vaccine and influenza infection, which indicate the interruption of PKR is not sufficient to eliminate eIF2α phosphorylation and other eIF2α kinase family members may compensate for loss of PKR functions.179 Perk−/− mice are viable, and the phenotypes involve strong changes of pancreatic β-cell secretory function and its survival. Therefore, Perk knockout animals have impaired glucose metabolism and develop early diabetes and growth retardation.180, 181 Perk−/− mice also exhibit severe osteopenia because PERK signaling is also important for the differentiation of osteoblasts.181–183 PERK signaling has divergent roles in neural tissues and cognitive function. Conditional disruption of PERK in the adult mouse forebrain was associated with altered behaviors.184
(3). EIF2α phosphatase gene knockout mice
Two somatic genes, Ppp1r15a and Ppp1r15b, encode the proteins GADD3464 and CReP,185 that recruit PP1 to form complex phosphatase to dephosphorylate peIF2α. The Ppp1r15a−/− mice have a normal phenotype; however, the ER stress response in their embryonic fibroblasts are different as compare to that of the wild type.186 In contrast, the Ppp1r15b−/− newborns exhibit severe growth retardation, impaired erythropoiesis, and none survives the first day of postnatal life.186
(4). eIF2α gene knock in mice
In all eukaryotic cells, eIF2α is part of a major assemblage in the initiation of protein translation. The phosphorylation at serine 51 interrupts normal function of eIF2α and causes global reduction of protein synthesis except ATF4 and CHOP.187 A knock in mouse model that has been developed by substitution serine 51 for alanine, creating a non-phosphorylatable eIF2α. Homozygous eIF2αS51A mutant mice die within 24 hours of birth due to impaired gluconeogenesis and hypoglycemia.188 Heterogenous eIF2αS51A mutant mice develop normally into adulthood. Nevertheless, theses mice become obese and glucose intolerant when fed a high-fat diet.189 Conditional destruction of eIF2α in pancreatic β-cell led to severe diabetic mice.190
(5). CHOP knock out mice
CHOP is a well-characterized downstream target of the eIF2α-ATF4 signaling branch. Deletion of Chop in mice does not alter animal survival and development. The literature using CHOP-deficient animals for disease models are broad, and here we feature a few studies. CHOP knockout mice have reduced cell apoptosis in lung and kidney induced by ER stressors.191 In the nervous system, exceptionally, CHOP knockout mice showed increased apoptosis in the hippocampal cells and decreased performance in memory-related behaviors.192 However, CHOP deficiency provides significant neuroprotection in the context of brain ischemia.193 Taken together, these selected studies suggest, at least in neural models, CHOP may play a protective role or enhance apoptosis depending on the disease context.
Conclusion and Perspectives
The ISR widely regulates cell survival and death via peIF2-ATF4 signaling and its downstream execution. The ISR contributes to many ocular diseases such as keratoconus, corneal dystrophy, HSV keratitis, cataract, DR, AMD, and glaucoma. The use of small molecules to counteract the peIF2α effects or target the eIF2α kinases/phosphatase is promising for treating ISR-related ocular disorders. However, drug selectivity and specificity need to be cautiously inspected. In vivo models such as gene knockout or knock-in mice are invaluable for studying ISR consequences in embryogenesis, development, and phenotypes. Together with ATF4 knockout mice that present with retarded lens development, future expansion of ocular ISR related disease models are mandatory for exploring potential therapeutic agents.
Supplementary Material
Acknowledgments
Funding:
This research was funded through a kind donation from the Niarchos fund (Wilmer Eye Institute) - JF, The Research to Prevent Blindness Unrestricted Grant (Wilmer Eye institute) – JF NIH T32 OD011089 (PI: Mankowski) – CP National Taiwan University Hospital Research Grant 108-M4386 - HSC
Footnotes
Declaration of interest:
The authors declare there is no conflict of interest.
References:
- 1.Costa-Mattioli M, Walter P. The integrated stress response: From mechanism to disease. Science. 2020;368(6489). doi: 10.1126/science.aat5314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pakos-Zebrucka K, Koryga I, Mnich K, Ljujic M, Samali A, Gorman AM. The integrated stress response. EMBO Rep. 2016;17(10):1374–1395. doi: 10.15252/embr.201642195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seo J, Fortuno ES 3rd, Suh JM, Stenesen D, Tang W, Parks EJ, Adams CM, Townes T, Graff JM. Atf4 regulates obesity, glucose homeostasis, and energy expenditure. Diabetes. 2009;58(11):2565–2573. doi: 10.2337/db09-0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harding HP, Novoa I, Zhang Y, Zeng H, Wek R, Schapira M, Ron D. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol Cell. 2000;6(5):1099–1108. doi: 10.1016/s1097-2765(00)00108-8. [DOI] [PubMed] [Google Scholar]
- 5.Ye J, Kumanova M, Hart LS, Sloane K, Zhang H, De Panis DN, Bobrovnikova-Marjon E, Diehl JA, Ron D, Koumenis C. The GCN2-ATF4 pathway is critical for tumour cell survival and proliferation in response to nutrient deprivation. EMBO J. 2010;29(12):2082–2096. doi: 10.1038/emboj.2010.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kilberg MS, Shan J, Su N. ATF4-dependent transcription mediates signaling of amino acid limitation. Trends Endocrinol Metab. 2009;20(9):436–443. doi: 10.1016/j.tem.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.B’Chir W, Maurin AC, Carraro V, Averous J, Jousse C, Muranishi Y, Parry L, Stepien G, Fafournoux P, Bruhat A. The eIF2alpha/ATF4 pathway is essential for stress-induced autophagy gene expression. Nucleic Acids Res. 2013;41(16):7683–7699. doi: 10.1093/nar/gkt563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen JJ, Throop MS, Gehrke L, Kuo I, Pal JK, Brodsky M, London IM. Cloning of the cDNA of the heme-regulated eukaryotic initiation factor 2 alpha (eIF-2 alpha) kinase of rabbit reticulocytes: homology to yeast GCN2 protein kinase and human double-stranded-RNA-dependent eIF-2 alpha kinase. Proc Natl Acad Sci U S A. 1991;88(17):7729–7733. doi: 10.1073/pnas.88.17.7729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clemens MJ, Elia A. The Double-Stranded RNA-Dependent Protein Kinase PKR: Structure and Function. J Interferon Cytokine Res. 1997;17(9):503–524. doi: 10.1089/jir.1997.17.503. [DOI] [PubMed] [Google Scholar]
- 10.Feldman DE, Chauhan V, Koong AC. The unfolded protein response: a novel component of the hypoxic stress response in tumors. Mol Cancer Res. 2005;3(11):597–605. doi: 10.1158/1541-7786.Mcr-05-0221. [DOI] [PubMed] [Google Scholar]
- 11.Harding HP, Zhang Y, Ron D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature. 1999;397(6716):271–274. doi: 10.1038/16729. [DOI] [PubMed] [Google Scholar]
- 12.Rutkowski DT, Arnold SM, Miller CN, Wu J, Li J, Gunnison KM, Mori K, Sadighi Akha AA, Raden D, Kaufman RJ. Adaptation to ER stress is mediated by differential stabilities of pro-survival and pro-apoptotic mRNAs and proteins. PLoS Biol. 2006;4(11):e374. doi: 10.1371/journal.pbio.0040374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bond S, Lopez-Lloreda C, Gannon PJ, Akay-Espinoza C, Jordan-Sciutto KL. The Integrated Stress Response and Phosphorylated Eukaryotic Initiation Factor 2α in Neurodegeneration. J Neuropathol Exp Neurol. 2020. doi: 10.1093/jnen/nlz129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ariyasu D, Yoshida H, Hasegawa Y. Endoplasmic Reticulum (ER) Stress and Endocrine Disorders. Int J Mol Sci. 2017;18(2). doi: 10.3390/ijms18020382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McConkey DJ. The integrated stress response and proteotoxicity in cancer therapy. Biochem Biophys Res Commun. 2017;482(3):450–453. doi: 10.1016/j.bbrc.2016.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oakes SA. Endoplasmic reticulum proteostasis: a key checkpoint in cancer. Am J Physiol Cell Physiol. 2017;312(2):C93–c102. doi: 10.1152/ajpcell.00266.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.So JS. Roles of Endoplasmic Reticulum Stress in Immune Responses. Mol Cells. 2018;41(8):705–716. doi: 10.14348/molcells.2018.0241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rodrigues L, Graca RSF, Carneiro LAM. Integrated Stress Responses to Bacterial Pathogenesis Patterns. Front Immunol. 2018;9:1306. doi: 10.3389/fimmu.2018.01306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bashir H, Seykora JT, Lee V. Invisible Shield: Review of the Corneal Epithelium as a Barrier to UV Radiation, Pathogens, and Other Environmental Stimuli. J Ophthalmic Vis Res. 2017;12(3):305–311. doi: 10.4103/jovr.jovr_114_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kroeger H, Chiang W-C, Felden J, Nguyen A, Lin JH. ER stress and unfolded protein response in ocular health and disease. FEBS J. 2019;286(2):399–412. doi: 10.1111/febs.14522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salminen A, Kauppinen A, Hyttinen JM, Toropainen E, Kaarniranta K. Endoplasmic reticulum stress in age-related macular degeneration: trigger for neovascularization. Mol Med. 2010;16(11–12):535–542. doi: 10.2119/molmed.2010.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ghosh R, Lipson KL, Sargent KE, Mercurio AM, Hunt JS, Ron D, Urano F. Transcriptional Regulation of VEGF-A by the Unfolded Protein Response Pathway. PLoS ONE. 2010;5(3):e9575. doi: 10.1371/journal.pone.0009575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Forrester J, Xu H. Good news–bad news: the Yin and Yang of immune privilege in the eye. Frontiers in Immunology. 2012;3(338). doi: 10.3389/fimmu.2012.00338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnston BP, McCormick C. Herpesviruses and the Unfolded Protein Response. Viruses. 2019;12(1): 10.3390/v12010017. doi: 10.3390/v12010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zode GS, Sharma AB, Lin X, Searby CC, Bugge K, Kim GH, Clark AF, Sheffield VC. Ocular-specific ER stress reduction rescues glaucoma in murine glucocorticoid-induced glaucoma. J Clin Invest. 2014;124(5):1956–1965. doi: 10.1172/JCI69774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li J, Wang JJ, Yu Q, Wang M, Zhang SX. Endoplasmic reticulum stress is implicated in retinal inflammation and diabetic retinopathy. FEBS Letters. 2009;583(9):1521–1527. doi: 10.1016/j.febslet.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang J, Zhou S, Gu J, Wang Y, Guo M, Liu Y. Differences in Unfolded Protein Response Pathway Activation in the Lenses of Three Types of Cataracts. PLoS One. 2015;10(6):e0130705. doi: 10.1371/journal.pone.0130705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coursey TG, Tukler Henriksson J, Barbosa FL, De Paiva CS, Pflugfelder SC. Interferon-γ–Induced Unfolded Protein Response in Conjunctival Goblet Cells as a Cause of Mucin Deficiency in Sjögren Syndrome. Am J Pathol. 2016;186(6):1547–1558. doi: 10.1016/j.ajpath.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Foster JW, Shinde V, Soiberman US, Sathe G, Liu S, Wan J, Qian J, Dauoud Y, Pandey A, Jun AS, et al. Integrated Stress Response and Decreased ECM in Cultured Stromal Cells From Keratoconus Corneas. Invest Ophthalmol Vis Sci. 2018;59(7):2977–2986. doi: 10.1167/iovs.18-24367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tanaka T, Tsujimura T, Takeda K, Sugihara A, Maekawa A, Terada N, Yoshida N, Akira S. Targeted disruption of ATF4 discloses its essential role in the formation of eye lens fibres. Genes Cells. 1998;3(12):801–810. doi: 10.1046/j.1365-2443.1998.00230.x. [DOI] [PubMed] [Google Scholar]
- 31.Starr CR, Gorbatyuk MS. Delineating the role of eIF2α in retinal degeneration. Cell Death Dis. 2019;10(6):409. doi: 10.1038/s41419-019-1641-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bhootada Y, Kotla P, Zolotukhin S, Gorbatyuk O, Bebok Z, Athar M, Gorbatyuk M. Limited ATF4 Expression in Degenerating Retinas with Ongoing ER Stress Promotes Photoreceptor Survival in a Mouse Model of Autosomal Dominant Retinitis Pigmentosa. PLoS One. 2016;11(5):e0154779. doi: 10.1371/journal.pone.0154779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singleton DC, Harris AL. Targeting the ATF4 pathway in cancer therapy. Expert Opin Ther Targets. 2012;16(12):1189–1202. doi: 10.1517/14728222.2012.728207. [DOI] [PubMed] [Google Scholar]
- 34.Hetz C, Chevet E, Harding HP. Targeting the unfolded protein response in disease. Nat Rev Drug Discov. 2013;12(9):703–719. doi: 10.1038/nrd3976. [DOI] [PubMed] [Google Scholar]
- 35.Joshi M, Kulkarni A, Pal JK. Small molecule modulators of eukaryotic initiation factor 2α kinases, the key regulators of protein synthesis. Biochimie. 2013;95(11):1980–1990. doi: 10.1016/j.biochi.2013.07.030. [DOI] [PubMed] [Google Scholar]
- 36.Ameri K, Harris AL. Activating transcription factor 4. Int J Biochem Cell Biol. 2008;40(1):14–21. doi: 10.1016/j.biocel.2007.01.020. [DOI] [PubMed] [Google Scholar]
- 37.Donnelly N, Gorman AM, Gupta S, Samali A. The eIF2alpha kinases: their structures and functions. Cell Mol Life Sci. 2013;70(19):3493–3511. doi: 10.1007/s00018-012-1252-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dever TE, Feng L, Wek RC, Cigan AM, Donahue TF, Hinnebusch AG. Phosphorylation of initiation factor 2 alpha by protein kinase GCN2 mediates gene-specific translational control of GCN4 in yeast. Cell. 1992;68(3):585–596. doi: 10.1016/0092-8674(92)90193-g. [DOI] [PubMed] [Google Scholar]
- 39.Kostura M, Mathews MB. Purification and activation of the double-stranded RNA-dependent eIF-2 kinase DAI. Mol Cell Biol. 1989;9(4):1576–1586. doi: 10.1128/mcb.9.4.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meurs E, Chong K, Galabru J, Thomas NS, Kerr IM, Williams BR, Hovanessian AG. Molecular cloning and characterization of the human double-stranded RNA-activated protein kinase induced by interferon. Cell. 1990;62(2):379–390. doi: 10.1016/0092-8674(90)90374-n. [DOI] [PubMed] [Google Scholar]
- 41.Shi Y, Vattem KM, Sood R, An J, Liang J, Stramm L, Wek RC. Identification and characterization of pancreatic eukaryotic initiation factor 2 alpha-subunit kinase, PEK, involved in translational control. Mol Cell Biol. 1998;18(12):7499–7509. doi: 10.1128/mcb.18.12.7499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Masson GR. Towards a model of GCN2 activation. Biochemical Society Transactions. 2019;47(5):1481–1488. doi: 10.1042/bst20190331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen J-J. Translational control by heme-regulated eIF2α kinase during erythropoiesis. Curr Opin Hematol. 2014;21(3):172–178. doi: 10.1097/moh.0000000000000030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Korennykh A, Walter P. Structural basis of the unfolded protein response. Annu Rev Cell Dev Biol. 2012;28:251–277. doi: 10.1146/annurev-cellbio-101011-155826. [DOI] [PubMed] [Google Scholar]
- 45.Hinnebusch AG. Molecular mechanism of scanning and start codon selection in eukaryotes. Microbiol Mol Biol Rev. 2011;75(3):434–467, first page of table of contents. doi: 10.1128/mmbr.00008-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kimball SR. Eukaryotic initiation factor eIF2. Int J Biochem Cell Biol. 1999;31(1):25–29. doi: 10.1016/s1357-2725(98)00128-9. [DOI] [PubMed] [Google Scholar]
- 47.Proud CG. eIF2 and the control of cell physiology. Semin Cell Dev Biol. 2005;16(1):3–12. doi: 10.1016/j.semcdb.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 48.Rowlands AG, Panniers R, Henshaw EC. The catalytic mechanism of guanine nucleotide exchange factor action and competitive inhibition by phosphorylated eukaryotic initiation factor 2. J Biol Chem. 1988;263(12):5526–5533. [PubMed] [Google Scholar]
- 49.Rabouw HH, Visser LJ, Passchier TC, Langereis MA, Liu F, Giansanti P, van Vliet ALW, Dekker JG, van der Grein SG, Saucedo JG, et al. Inhibition of the integrated stress response by viral proteins that block p-eIF2–eIF2B association. Nat Microbiol. 2020;5(11):1361–1373. doi: 10.1038/s41564-020-0759-0. [DOI] [PubMed] [Google Scholar]
- 50.Harding HP, Zhang Y, Zeng H, Novoa I, Lu PD, Calfon M, Sadri N, Yun C, Popko B, Paules R, et al. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell. 2003;11(3):619–633. doi: 10.1016/s1097-2765(03)00105-9. [DOI] [PubMed] [Google Scholar]
- 51.Han AP. Heme-regulated eIF2alpha kinase (HRI) is required for translational regulation and survival of erythroid precursors in iron deficiency. The EMBO Journal. 2001;20(23):6909–6918. doi: 10.1093/emboj/20.23.6909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Balachandran S, Roberts PC, Brown LE, Truong H, Pattnaik AK, Archer DR, Barber GN. Essential role for the dsRNA-dependent protein kinase PKR in innate immunity to viral infection. Immunity. 2000;13(1):129–141. doi: 10.1016/s1074-7613(00)00014-5. [DOI] [PubMed] [Google Scholar]
- 53.Garcia MA, Meurs EF, Esteban M. The dsRNA protein kinase PKR: virus and cell control. Biochimie. 2007;89(6–7):799–811. doi: 10.1016/j.biochi.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 54.Ron D Translational control in the endoplasmic reticulum stress response. J Clin Invest. 2002;110(10):1383–1388. doi: 10.1172/JCI16784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chan CP, Kok KH, Tang HM, Wong CM, Jin DY. Internal ribosome entry site-mediated translational regulation of ATF4 splice variant in mammalian unfolded protein response. Biochim Biophys Acta. 2013;1833(10):2165–2175. doi: 10.1016/j.bbamcr.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 56.Palam LR, Baird TD, Wek RC. Phosphorylation of eIF2 facilitates ribosomal bypass of an inhibitory upstream ORF to enhance CHOP translation. J Biol Chem. 2011;286(13):10939–10949. doi: 10.1074/jbc.M110.216093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee YY, Cevallos RC, Jan E. An upstream open reading frame regulates translation of GADD34 during cellular stresses that induce eIF2alpha phosphorylation. J Biol Chem. 2009;284(11):6661–6673. doi: 10.1074/jbc.M806735200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Harding HP, Zhang Y, Zeng H, Novoa I, Lu PD, Calfon M, Sadri N, Yun C, Popko B, Paules R, et al. An Integrated Stress Response Regulates Amino Acid Metabolism and Resistance to Oxidative Stress. Mol Cell. 2003;11(3):619–633. doi: 10.1016/s1097-2765(03)00105-9. [DOI] [PubMed] [Google Scholar]
- 59.Yoshizawa T, Hinoi E, Jung DY, Kajimura D, Ferron M, Seo J, Graff JM, Kim JK, Karsenty G. The transcription factor ATF4 regulates glucose metabolism in mice through its expression in osteoblasts. J Clin Invest. 2009;119(9):2807–2817. doi: 10.1172/jci39366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bretin A, Carriere J, Dalmasso G, Bergougnoux A, B’Chir W, Maurin AC, Muller S, Seibold F, Barnich N, Bruhat A, et al. Activation of the EIF2AK4-EIF2A/eIF2alpha-ATF4 pathway triggers autophagy response to Crohn disease-associated adherent-invasive Escherichia coli infection. Autophagy. 2016;12(5):770–783. doi: 10.1080/15548627.2016.1156823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang Y, Ning Y, Alam GN, Jankowski BM, Dong Z, Nor JE, Polverini PJ. Amino acid deprivation promotes tumor angiogenesis through the GCN2/ATF4 pathway. Neoplasia. 2013;15(8):989–997. doi: 10.1593/neo.13262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wortel IMN, Van Der Meer LT, Kilberg MS, Van Leeuwen FN. Surviving Stress: Modulation of ATF4-Mediated Stress Responses in Normal and Malignant Cells. Trends Endocrinol Metab. 2017;28(11):794–806. doi: 10.1016/j.tem.2017.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jousse C, Oyadomari S, Novoa I, Lu P, Zhang Y, Harding HP, Ron D. Inhibition of a constitutive translation initiation factor 2alpha phosphatase, CReP, promotes survival of stressed cells. J Cell Biol. 2003;163(4):767–775. doi: 10.1083/jcb.200308075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Novoa I, Zeng H, Harding HP, Ron D. Feedback inhibition of the unfolded protein response by GADD34-mediated dephosphorylation of eIF2alpha. J Cell Biol. 2001;153(5):1011–1022. doi: 10.1083/jcb.153.5.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Marciniak SJ, Yun CY, Oyadomari S, Novoa I, Zhang Y, Jungreis R, Nagata K, Harding HP, Ron D. CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev. 2004;18(24):3066–3077. doi: 10.1101/gad.1250704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Han J, Back SH, Hur J, Lin Y-H, Gildersleeve R, Shan J, Yuan CL, Krokowski D, Wang S, Hatzoglou M, et al. ER-stress-induced transcriptional regulation increases protein synthesis leading to cell death. Nat Cell Biol. 2013;15(5):481–490. doi: 10.1038/ncb2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Frank CL, Ge X, Xie Z, Zhou Y, Tsai LH. Control of Activating Transcription Factor 4 (ATF4) Persistence by Multisite Phosphorylation Impacts Cell Cycle Progression and Neurogenesis. J Biol Chem. 2010;285(43):33324–33337. doi: 10.1074/jbc.m110.140699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lin H-K, Chen Z, Wang G, Nardella C, Lee S-W, Chan C-H, Yang W-L, Wang J, Egia A, Nakayama KI, et al. Skp2 targeting suppresses tumorigenesis by Arf-p53-independent cellular senescence. Nature. 2010;464(7287):374–379. doi: 10.1038/nature08815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jun AS. One hundred years of Fuchs’ dystrophy. Ophthalmology. 2010;117(5):859–860 e814. doi: 10.1016/j.ophtha.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 70.Eghrari AO, Riazuddin SA, Gottsch JD. Fuchs Corneal Dystrophy. Prog Mol Biol Transl Sci. 2015;134:79–97. doi: 10.1016/bs.pmbts.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 71.Engler C, Kelliher C, Spitze AR, Speck CL, Eberhart CG, Jun AS. Unfolded protein response in fuchs endothelial corneal dystrophy: a unifying pathogenic pathway? Am J Ophthalmol. 2010;149(2):194–202.e192. doi: 10.1016/j.ajo.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Okumura N, Kitahara M, Okuda H, Hashimoto K, Ueda E, Nakahara M, Kinoshita S, Young RD, Quantock AJ, Tourtas T, et al. Sustained Activation of the Unfolded Protein Response Induces Cell Death in Fuchs’ Endothelial Corneal Dystrophy. Invest Ophthalmol Vis Sci. 2017;58(9):3697–3707. doi: 10.1167/iovs.16-21023. [DOI] [PubMed] [Google Scholar]
- 73.Ferrari G, Rama P. The keratoconus enigma: A pathogenesis review. Ocul Surf. 2020. July;18(3):363–373 doi: 10.1016/j.jtos.2020.03.006. [DOI] [PubMed] [Google Scholar]
- 74.Chaerkady R, Shao H, Scott SG, Pandey A, Jun AS, Chakravarti S. The keratoconus corneal proteome: loss of epithelial integrity and stromal degeneration. J Proteomics. 2013;87:122–131. doi: 10.1016/j.jprot.2013.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Klintworth GK. Advances in the molecular genetics of corneal dystrophies. Am J Ophthalmol. 1999;128(6):747–754. doi: 10.1016/s0002-9394(99)00358-x. [DOI] [PubMed] [Google Scholar]
- 76.Choi SI, Lee E, Jeong JB, Akuzum B, Maeng YS, Kim TI, Kim EK. 4-Phenylbutyric acid reduces mutant-TGFBIp levels and ER stress through activation of ERAD pathway in corneal fibroblasts of granular corneal dystrophy type 2. Biochem Biophys Res Commun. 2016;477(4):841–846. doi: 10.1016/j.bbrc.2016.06.146. [DOI] [PubMed] [Google Scholar]
- 77.Shukla D, Farooq, Shah. The role of herpesviruses in ocular infections. Virus Adaptation and Treatment. 2010;2:115–123. doi: 10.2147/vaat.s9500. [DOI] [Google Scholar]
- 78.Valerio GS, Lin CC. Ocular manifestations of herpes simplex virus. Curr Opin Ophthalmol. 2019;30(6):525–531. doi: 10.1097/icu.0000000000000618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Burnett HF, Audas TE, Liang G, Lu RR. Herpes simplex virus-1 disarms the unfolded protein response in the early stages of infection. Cell Stress Chaperones. 2012;17(4):473–483. doi: 10.1007/s12192-012-0324-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Poppers J, Mulvey M, Khoo D, Mohr I. Inhibition of PKR activation by the proline-rich RNA binding domain of the herpes simplex virus type 1 Us11 protein. J Virol. 2000;74(23):11215–11221. doi: 10.1128/jvi.74.23.11215-11221.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.He B, Gross M, Roizman B. The gamma(1)34.5 protein of herpes simplex virus 1 complexes with protein phosphatase 1alpha to dephosphorylate the alpha subunit of the eukaryotic translation initiation factor 2 and preclude the shutoff of protein synthesis by double-stranded RNA-activated protein kinase. Proc Natl Acad Sci U S A. 1997;94(3):843–848. doi: 10.1073/pnas.94.3.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Charron AJ, Ward SL, North BJ, Ceron S, Leib DA. The US11 Gene of Herpes Simplex Virus 1 Promotes Neuroinvasion and Periocular Replication following Corneal Infection. J Virol. 2019;93(9):e02246. doi: 10.1128/JVI.02246-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hejtmancik JF, Riazuddin SA, McGreal R, Liu W, Cvekl A, Shiels A. Lens Biology and Biochemistry. Prog Mol Biol Transl Sci. 2015;134(169–201. doi: 10.1016/bs.pmbts.2015.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Graw J Eye development. Curr Top Dev Biol. 2010;90(343–386. doi: 10.1016/s0070-2153(10)90010-0. [DOI] [PubMed] [Google Scholar]
- 85.Brennan LA, Kantorow WL, Chauss D, McGreal R, He S, Mattucci L, Wei J, Riazuddin SA, Cvekl A, Hejtmancik JF, et al. Spatial expression patterns of autophagy genes in the eye lens and induction of autophagy in lens cells. Mol Vis. 2012;18:1773–1786. [PMC free article] [PubMed] [Google Scholar]
- 86.Khan SY, Ali M, Kabir F, Renuse S, Na CH, Talbot CC Jr., Hackett SF, Riazuddin SA. Proteome Profiling of Developing Murine Lens Through Mass Spectrometry. Invest Ophthalmol Vis Sci. 2018;59(1):100–107. doi: 10.1167/iovs.17-21601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Khairallah M, Kahloun R, Bourne R, Limburg H, Flaxman SR, Jonas JB, Keeffe J, Leasher J, Naidoo K, Pesudovs K, et al. Number of People Blind or Visually Impaired by Cataract Worldwide and in World Regions, 1990 to 2010. Invest Ophthalmol Vis Sci. 2015;56(11):6762–6769. doi: 10.1167/iovs.15-17201. [DOI] [PubMed] [Google Scholar]
- 88.Bron AJ, Vrensen GF, Koretz J, Maraini G, Harding JJ. The ageing lens. Ophthalmologica. 2000;214(1):86–104. doi: 10.1159/000027475. [DOI] [PubMed] [Google Scholar]
- 89.Tang HZ, Yang LM. Activation of the unfolded protein response in aged human lenses. Mol Med Rep. 2015;12(1):389–393. doi: 10.3892/mmr.2015.3417. [DOI] [PubMed] [Google Scholar]
- 90.Yang J, Zhou S, Gu J, Guo M, Xia H, Liu Y. UPR Activation and the Down-Regulation of alpha-Crystallin in Human High Myopia-Related Cataract Lens Epithelium. PLoS One. 2015;10(9):e0137582. doi: 10.1371/journal.pone.0137582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ikesugi K, Yamamoto R, Mulhern ML, Shinohara T. Role of the unfolded protein response (UPR) in cataract formation. Exp Eye Res. 2006;83(3):508–516. doi: 10.1016/j.exer.2006.01.033. [DOI] [PubMed] [Google Scholar]
- 92.Kroeger H, Chiang WC, Felden J, Nguyen A, Lin JH. ER stress and unfolded protein response in ocular health and disease. FEBS J. 2019;286(2):399–412. doi: 10.1111/febs.14522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kobrin Klein BE. Overview of Epidemiologic Studies of Diabetic Retinopathy. Ophthalmic Epidemiol. 2007;14(4):179–183. doi: 10.1080/09286580701396720. [DOI] [PubMed] [Google Scholar]
- 94.Ejaz S Importance of pericytes and mechanisms of pericyte loss during diabetes retinopathy. Diabetes, Obes Metab. 2008;10(1):53–63. doi: 10.1111/j.1463-1326.2007.00795.x. [DOI] [PubMed] [Google Scholar]
- 95.Zhong Y, Wang JJ, Zhang SX. Intermittent but not constant high glucose induces ER stress and inflammation in human retinal pericytes. Adv Exp Med Biol. 2012;723:285–292. doi: 10.1007/978-1-4614-0631-0_37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mitchell P, Liew G, Gopinath B, Wong TY. Age-related macular degeneration. The Lancet. 2018;392(10153):1147–1159. doi: 10.1016/s0140-6736(18)31550-2. [DOI] [PubMed] [Google Scholar]
- 97.Gehrs KM, Anderson DH, Johnson LV, Hageman GS. Age‐related macular degeneration—emerging pathogenetic and therapeutic concepts. Ann Med. 2006;38(7):450–471. doi: 10.1080/07853890600946724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Roybal CN, Hunsaker LA, Barbash O, Vander Jagt DL, Abcouwer SF. The oxidative stressor arsenite activates vascular endothelial growth factor mRNA transcription by an ATF4-dependent mechanism. J Biol Chem. 2005;280(21):20331–20339. doi: 10.1074/jbc.M411275200. [DOI] [PubMed] [Google Scholar]
- 99.Pollreisz A, Afonyushkin T, Oskolkova OV, Gruber F, Bochkov VN, Schmidt-Erfurth U. Retinal pigment epithelium cells produce VEGF in response to oxidized phospholipids through mechanisms involving ATF4 and protein kinase CK2. Exp Eye Res. 2013;116:177–184. doi: 10.1016/j.exer.2013.08.021. [DOI] [PubMed] [Google Scholar]
- 100.Weinreb RN, Aung T, Medeiros FA. The Pathophysiology and Treatment of Glaucoma. JAMA. 2014;311(18):1901. doi: 10.1001/jama.2014.3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zode GS, Kuehn MH, Nishimura DY, Searby CC, Mohan K, Grozdanic SD, Bugge K, Anderson MG, Clark AF, Stone EM, et al. Reduction of ER stress via a chemical chaperone prevents disease phenotypes in a mouse model of primary open angle glaucoma. J Clin Invest. 2011;121(9):3542–3553. doi: 10.1172/JCI58183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Peters JC, Bhattacharya S, Clark AF, Zode GS. Increased Endoplasmic Reticulum Stress in Human Glaucomatous Trabecular Meshwork Cells and Tissues. Invest Ophthalmol Vis Sci. 2015;56(6):3860–3868. doi: 10.1167/iovs.14-16220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Foufelle F, Fromenty B. Role of endoplasmic reticulum stress in drug-induced toxicity. Pharmacol Res Perspect. 2016;4(1):e00211. doi: 10.1002/prp2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Srivastava RK, Li C, Ahmad A, Abrams O, Gorbatyuk MS, Harrod KS, Wek RC, Afaq F, Athar M. ATF4 regulates arsenic trioxide-mediated NADPH oxidase, ER-mitochondrial crosstalk and apoptosis. Arch Biochem Biophys. 2016;609:39–50. doi: 10.1016/j.abb.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Carlberg M, Dricu A, Blegen H, Kass GEN, Orrenius S, Larsson O. Short exposures to tunicamycin induce apoptosis in SV40-transformed but not in normal human fibroblasts. Carcinogenesis. 1996;17(12):2589–2596. doi: 10.1093/carcin/17.12.2589. [DOI] [PubMed] [Google Scholar]
- 106.Treiman M, Caspersen C, Christensen SB. A tool coming of age: thapsigargin as an inhibitor of sarco-endoplasmic reticulum Ca(2+)-ATPases. Trends Pharmacol Sci. 1998;19(4):131–135. doi: 10.1016/s0165-6147(98)01184-5. [DOI] [PubMed] [Google Scholar]
- 107.Shi K, Wang D, Cao X, Ge Y. Endoplasmic Reticulum Stress Signaling Is Involved in Mitomycin C(MMC)-Induced Apoptosis in Human Fibroblasts via PERK Pathway. PLoS ONE. 2013;8(3):e59330. doi: 10.1371/journal.pone.0059330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Martinez VD, Vucic EA, Becker-Santos DD, Gil L, Lam WL. Arsenic exposure and the induction of human cancers. J Toxicol. 2011;2011:431287. doi: 10.1155/2011/431287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Guo Q, Li H, Liu J, Xu L, Yang L, Sun Z, Zhou B. Tunicamycin aggravates endoplasmic reticulum stress and airway inflammation via PERK-ATF4-CHOP signaling in a murine model of neutrophilic asthma. J Asthma. 2017;54(2):125–133. doi: 10.1080/02770903.2016.1205085. [DOI] [PubMed] [Google Scholar]
- 110.Shaabani N, Honke N, Lang PA, Gorg B, Proksch P, Gailus N, Gotoh T, Haussinger D, Lang KS. Tunicamycin inhibits diabetes. Cell Physiol Biochem. 2012;29(3–4):595–602. doi: 10.1159/000338513. [DOI] [PubMed] [Google Scholar]
- 111.Shehadeh-Mashor R, Srinivasan S, Boimer C, Lee K, Tomkins O, Slomovic AR. Management of recurrent pterygium with intraoperative mitomycin C and conjunctival autograft with fibrin glue. Am J Ophthalmol. 2011;152(5):730–732. doi: 10.1016/j.ajo.2011.04.034. [DOI] [PubMed] [Google Scholar]
- 112.Katz GJ, Higginbotham EJ, Lichter PR, Skuta GL, Musch DC, Bergstrom TJ, Johnson AT. Mitomycin C versus 5-fluorouracil in high-risk glaucoma filtering surgery. Extended follow-up. Ophthalmology. 1995;102(9):1263–1269. doi: 10.1016/s0161-6420(95)30875-5. [DOI] [PubMed] [Google Scholar]
- 113.Doultsinos D, Avril T, Lhomond S, Dejeans N, Guedat P, Chevet E. Control of the Unfolded Protein Response in Health and Disease. SLAS Discov. 2017;22(7):787–800. doi: 10.1177/2472555217701685. [DOI] [PubMed] [Google Scholar]
- 114.Hetz C, Axten JM, Patterson JB. Pharmacological targeting of the unfolded protein response for disease intervention. Nat Chem Biol. 2019;15(8):764–775. doi: 10.1038/s41589-019-0326-2. [DOI] [PubMed] [Google Scholar]
- 115.Rabouw HH, Langereis MA, Anand AA, Visser LJ, De Groot RJ, Walter P, Van Kuppeveld FJM. Small molecule ISRIB suppresses the integrated stress response within a defined window of activation. Proceedings of the National Academy of Sciences. 2019;116(6):2097–2102. doi: 10.1073/pnas.1815767116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sidrauski C, Acosta-Alvear D, Khoutorsky A, Vedantham P, Hearn BR, Li H, Gamache K, Gallagher CM, Ang KK, Wilson C, et al. Pharmacological brake-release of mRNA translation enhances cognitive memory. Elife. 2013;2:e00498. doi: 10.7554/eLife.00498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sidrauski C, Tsai JC, Kampmann M, Hearn BR, Vedantham P, Jaishankar P, Sokabe M, Mendez AS, Newton BW, Tang EL, et al. Pharmacological dimerization and activation of the exchange factor eIF2B antagonizes the integrated stress response. Elife. 2015;4:e07314. doi: 10.7554/eLife.07314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hearn BR, Jaishankar P, Sidrauski C, Tsai JC, Vedantham P, Fontaine SD, Walter P, Renslo AR. Structure-Activity Studies of Bis-O-Arylglycolamides: Inhibitors of the Integrated Stress Response. ChemMedChem. 2016;11(8):870–880. doi: 10.1002/cmdc.201500483. [DOI] [PubMed] [Google Scholar]
- 119.Bugallo R, Marlin E, Baltanas A, Toledo E, Ferrero R, Vinueza-Gavilanes R, Larrea L, Arrasate M, Aragon T. Fine tuning of the unfolded protein response by ISRIB improves neuronal survival in a model of amyotrophic lateral sclerosis. Cell Death Dis. 2020;11(5):397. doi: 10.1038/s41419-020-2601-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Halliday M, Radford H, Sekine Y, Moreno J, Verity N, Le Quesne J, Ortori CA, Barrett DA, Fromont C, Fischer PM, et al. Partial restoration of protein synthesis rates by the small molecule ISRIB prevents neurodegeneration without pancreatic toxicity. Cell Death Dis. 2015;6(3):e1672-e1672. doi: 10.1038/cddis.2015.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chou A, Krukowski K, Jopson T, Zhu PJ, Costa-Mattioli M, Walter P, Rosi S. Inhibition of the integrated stress response reverses cognitive deficits after traumatic brain injury. Proc Natl Acad Sci U S A. 2017;114(31):E6420–E6426. doi: 10.1073/pnas.1707661114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhu PJ, Khatiwada S, Cui Y, Reineke LC, Dooling SW, Kim JJ, Li W, Walter P, Costa-Mattioli M. Activation of the ISR mediates the behavioral and neurophysiological abnormalities in Down syndrome. Science. 2019;366(6467):843–849. doi: 10.1126/science.aaw5185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Soiberman US, Shehata AEM, Lu MX, Young T, Daoud YJ, Chakravarti S, Jun AS, Foster JW. Small Molecule Modulation of the Integrated Stress Response Governs the Keratoconic Phenotype In Vitro. Invest Ophthalmol Vis Sci. 2019;60(10):3422–3431. doi: 10.1167/iovs.19-27151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sundrud MS, Koralov SB, Feuerer M, Calado DP, Kozhaya AE, Rhule-Smith A, Lefebvre RE, Unutmaz D, Mazitschek R, Waldner H, et al. Halofuginone inhibits TH17 cell differentiation by activating the amino acid starvation response. Science. 2009;324(5932):1334–1338. doi: 10.1126/science.1172638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bunpo P, Dudley A, Cundiff JK, Cavener DR, Wek RC, Anthony TG. GCN2 protein kinase is required to activate amino acid deprivation responses in mice treated with the anti-cancer agent L-asparaginase. J Biol Chem. 2009;284(47):32742–32749. doi: 10.1074/jbc.M109.047910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Long Y, Tsai WB, Wangpaichitr M, Tsukamoto T, Savaraj N, Feun LG, Kuo MT. Arginine deiminase resistance in melanoma cells is associated with metabolic reprogramming, glucose dependence, and glutamine addiction. Mol Cancer Ther. 2013;12(11):2581–2590. doi: 10.1158/1535-7163.MCT-13-0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Chen T, Ozel D, Qiao Y, Harbinski F, Chen L, Denoyelle S, He X, Zvereva N, Supko JG, Chorev M, et al. Chemical genetics identify eIF2alpha kinase heme-regulated inhibitor as an anticancer target. Nat Chem Biol. 2011;7(9):610–616. doi: 10.1038/nchembio.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hu W, Hofstetter W, Wei X, Guo W, Zhou Y, Pataer A, Li H, Fang B, Swisher SG. Double-stranded RNA-dependent protein kinase-dependent apoptosis induction by a novel small compound. J Pharmacol Exp Ther. 2009;328(3):866–872. doi: 10.1124/jpet.108.141754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ma C-H, Wu C-H, Jou IM, Tu Y-K, Hung C-H, Chou W-C, Chang Y-C, Hsieh P-L, Tsai K-L. PKR Promotes Oxidative Stress and Apoptosis of Human Articular Chondrocytes by Causing Mitochondrial Dysfunction through p38 MAPK Activation—PKR Activation Causes Apoptosis in Human Chondrocytes. Antioxidants. 2019;8(9):370. doi: 10.3390/antiox8090370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Stockwell SR, Platt G, Barrie SE, Zoumpoulidou G, Te Poele RH, Aherne GW, Wilson SC, Sheldrake P, McDonald E, Venet M, et al. Mechanism-based screen for G1/S checkpoint activators identifies a selective activator of EIF2AK3/PERK signalling. PLoS One. 2012;7(1):e28568. doi: 10.1371/journal.pone.0028568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Atkins C, Liu Q, Minthorn E, Zhang SY, Figueroa DJ, Moss K, Stanley TB, Sanders B, Goetz A, Gaul N, et al. Characterization of a novel PERK kinase inhibitor with antitumor and antiangiogenic activity. Cancer Res. 2013;73(6):1993–2002. doi: 10.1158/0008-5472.Can-12-3109. [DOI] [PubMed] [Google Scholar]
- 132.Elkin M Halofuginone: a potent inhibitor of critical steps in angiogenesis progression. 2000;14(15):2477–2485. doi: 10.1096/fj.00-0292com. [DOI] [PubMed] [Google Scholar]
- 133.Yang L, Tan D, Piao H. Myelin Basic Protein Citrullination in Multiple Sclerosis: A Potential Therapeutic Target for the Pathology. Neurochem Res. 2016;41(8):1845–1856. doi: 10.1007/s11064-016-1920-2. [DOI] [PubMed] [Google Scholar]
- 134.Bonilha VL, Shadrach KG, Rayborn ME, Li Y, Pauer GJT, Hagstrom SA, Bhattacharya SK, Hollyfield JG. Retinal deimination and PAD2 levels in retinas from donors with age-related macular degeneration (AMD).Exp Eye Res 2013;111:71–78. doi: 10.1016/j.exer.2013.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Bhattacharya SK, Crabb JS, Bonilha VL, Gu X, Takahara H, Crabb JW. Proteomics implicates peptidyl arginine deiminase 2 and optic nerve citrullination in glaucoma pathogenesis. Invest Ophthalmol Vis Sci. 2006;47(6):2508–2514. doi: 10.1167/iovs.05-1499. [DOI] [PubMed] [Google Scholar]
- 136.Park JH, Baron S. Herpetic keratoconjunctivitis: therapy with synthetic double-stranded RNA. Science. 1968;162(3855):811–813. doi: 10.1126/science.162.3855.811. [DOI] [PubMed] [Google Scholar]
- 137.Kaufman HE, Ellison ED, Waltman SR. Double-stranded RNA, an interferon inducer, in herpes simplex keratitis. Am J Ophthalmol. 1969;68(3):486–491. doi: 10.1016/0002-9394(69)90720-x. [DOI] [PubMed] [Google Scholar]
- 138.Robert F, Williams C, Yan Y, Donohue E, Cencic R, Burley SK, Pelletier J. Blocking UV-induced eIF2alpha phosphorylation with small molecule inhibitors of GCN2. Chem Biol Drug Des. 2009;74(1):57–67. doi: 10.1111/j.1747-0285.2009.00827.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Rosen MD, Woods CR, Goldberg SD, Hack MD, Bounds AD, Yang Y, Wagaman PC, Phuong VK, Ameriks AP, Barrett TD, et al. Discovery of the first known small-molecule inhibitors of heme-regulated eukaryotic initiation factor 2alpha (HRI) kinase. Bioorg Med Chem Lett. 2009;19(23):6548–6551. doi: 10.1016/j.bmcl.2009.10.033. [DOI] [PubMed] [Google Scholar]
- 140.Jammi NV, Whitby LR, Beal PA. Small molecule inhibitors of the RNA-dependent protein kinase. Biochem Biophys Res Commun. 2003;308(1):50–57. doi: 10.1016/s0006-291x(03)01318-4. [DOI] [PubMed] [Google Scholar]
- 141.Hu Y, Conway TW. 2-Aminopurine inhibits the double-stranded RNA-dependent protein kinase both in vitro and in vivo. J Interferon Res. 1993;13(5):323–328. doi: 10.1089/jir.1993.13.323. [DOI] [PubMed] [Google Scholar]
- 142.Axten JM, Medina JR, Feng Y, Shu A, Romeril SP, Grant SW, Li WH, Heerding DA, Minthorn E, Mencken T, et al. Discovery of 7-methyl-5-(1-{[3-(trifluoromethyl)phenyl]acetyl}−2,3-dihydro-1H-indol-5-yl)-7H-p yrrolo[2,3-d]pyrimidin-4-amine (GSK2606414), a potent and selective first-in-class inhibitor of protein kinase R (PKR)-like endoplasmic reticulum kinase (PERK). J Med Chem. 2012;55(16):7193–7207. doi: 10.1021/jm300713s. [DOI] [PubMed] [Google Scholar]
- 143.Axten JM, Romeril SP, Shu A, Ralph J, Medina JR, Feng Y, Li WH, Grant SW, Heerding DA, Minthorn E, et al. Discovery of GSK2656157: An Optimized PERK Inhibitor Selected for Preclinical Development. ACS Med Chem Lett. 2013;4(10):964–968. doi: 10.1021/ml400228e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Jiang X, Wei Y, Zhang T, Zhang Z, Qiu S, Zhou X, Zhang S. Effects of GSK2606414 on cell proliferation and endoplasmic reticulum stressassociated gene expression in retinal pigment epithelial cells. Mol Med Rep. 2017;15(5):3105–3110. doi: 10.3892/mmr.2017.6418. [DOI] [PubMed] [Google Scholar]
- 145.Kojima E, Takeuchi A, Haneda M, Yagi F, Hasegawa T, Yamaki K-I, Takeda K, Akira S, Shimokata K, Isobe K-I. The function of GADD34 is a recovery from a shutoff of protein synthesis induced by ER stress—elucidation by GADD34‐deficient mice. The FASEB Journal. 2003;17(11):1–18. doi: 10.1096/fj.02-1184fje. [DOI] [PubMed] [Google Scholar]
- 146.Tsaytler P, Bertolotti A. Exploiting the selectivity of protein phosphatase 1 for pharmacological intervention. FEBS Journal. 2013;280(2):766–770. doi: 10.1111/j.1742-4658.2012.08535.x. [DOI] [PubMed] [Google Scholar]
- 147.De Gassart A, Bujisic B, Zaffalon L, Decosterd LA, Di Micco A, Frera G, Tallant R, Martinon F. An inhibitor of HIV-1 protease modulates constitutive eIF2alpha dephosphorylation to trigger a specific integrated stress response. Proc Natl Acad Sci U S A. 2016;113(2):E117–126. doi: 10.1073/pnas.1514076113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Boyce M, Bryant KF, Jousse C, Long K, Harding HP, Scheuner D, Kaufman RJ, Ma D, Coen DM, Ron D, et al. A selective inhibitor of eIF2alpha dephosphorylation protects cells from ER stress. Science. 2005;307(5711):935–939. doi: 10.1126/science.1101902. [DOI] [PubMed] [Google Scholar]
- 149.Bryant KF, Macari ER, Malik N, Boyce M, Yuan J, Coen DM. ICP34.5-dependent and -independent activities of salubrinal in herpes simplex virus-1 infected cells. Virology. 2008;379(2):197–204. doi: 10.1016/j.virol.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wang Y, Osakue D, Yang E, Zhou Y, Gong H, Xia X, Du Y. Endoplasmic Reticulum Stress Response of Trabecular Meshwork Stem Cells and Trabecular Meshwork Cells and Protective Effects of Activated PERK Pathway. Invest Ophthalmol Vis Sci. 2019;60(1):265–273. doi: 10.1167/iovs.18-25477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hirai S, Kurashima H, Nakamura D, Komatsu T, Yasuda Y, Habashita-Obata S, Ichikawa S, Katsuta O, Iwawaki T, Kohno K. 2-Phenyl-APB-144-Induced Retinal Pigment Epithelium Degeneration and Its Underlying Mechanisms. J Ocul Pharmacol Ther. 2015;31(9):570–584. doi: 10.1089/jop.2014.0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zhang L, Xia Q, Zhou Y, Li J. Endoplasmic reticulum stress and autophagy contribute to cadmium-induced cytotoxicity in retinal pigment epithelial cells. Toxicol Lett. 2019;311:105–113. doi: 10.1016/j.toxlet.2019.05.001. [DOI] [PubMed] [Google Scholar]
- 153.Imming P, Sinning C, Meyer A. Drugs, their targets and the nature and number of drug targets. Nat Rev Drug Discov. 2006;5(10):821–834. doi: 10.1038/nrd2132. [DOI] [PubMed] [Google Scholar]
- 154.Elefteriou F, Ahn JD, Takeda S, Starbuck M, Yang X, Liu X, Kondo H, Richards WG, Bannon TW, Noda M, et al. Leptin regulation of bone resorption by the sympathetic nervous system and CART. Nature. 2005;434(7032):514–520. doi: 10.1038/nature03398. [DOI] [PubMed] [Google Scholar]
- 155.Yang X, Matsuda K, Bialek P, Jacquot S, Masuoka HC, Schinke T, Li L, Brancorsini S, Sassone-Corsi P, Townes TM, et al. ATF4 is a substrate of RSK2 and an essential regulator of osteoblast biology; implication for Coffin-Lowry Syndrome. Cell. 2004;117(3):387–398. doi: 10.1016/s0092-8674(04)00344-7. [DOI] [PubMed] [Google Scholar]
- 156.Cohen MS, Zhang C, Shokat KM, Taunton J. Structural bioinformatics-based design of selective, irreversible kinase inhibitors. Science. 2005;308(5726):1318–1321. doi: 10.1126/science1108367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Sapkota GP, Cummings L, Newell FS, Armstrong C, Bain J, Frodin M, Grauert M, Hoffmann M, Schnapp G, Steegmaier M, et al. BI-D1870 is a specific inhibitor of the p90 RSK (ribosomal S6 kinase) isoforms in vitro and in vivo. Biochem J. 2007;401(1):29–38. doi: 10.1042/bj20061088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Smith JA, Poteet-Smith CE, Xu Y, Errington TM, Hecht SM, Lannigan DA. Identification of the first specific inhibitor of p90 ribosomal S6 kinase (RSK) reveals an unexpected role for RSK in cancer cell proliferation. Cancer Res. 2005;65(3):1027–1034. [PubMed] [Google Scholar]
- 159.Murray AJ. Pharmacological PKA inhibition: all may not be what it seems. Sci Signal. 2008;1(22):re4. doi: 10.1126/scisignal.122re4. [DOI] [PubMed] [Google Scholar]
- 160.Brudvik KW, Paulsen JE, Aandahl EM, Roald B, Tasken K. Protein kinase A antagonist inhibits beta-catenin nuclear translocation, c-Myc and COX-2 expression and tumor promotion in Apc(Min/+) mice. Mol Cancer. 2011;10:149. doi: 10.1186/1476-4598-10-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Fenton TR, Gout IT. Functions and regulation of the 70kDa ribosomal S6 kinases. Int J Biochem Cell Biol. 2011;43(1):47–59. doi: 10.1016/j.biocel.2010.09.018. [DOI] [PubMed] [Google Scholar]
- 162.Kotani T Protein kinase A activity and Hedgehog signaling pathway. Vitam Horm. 2012;88:273–291. doi: 10.1016/B978-0-12-394622-5.00012-2. [DOI] [PubMed] [Google Scholar]
- 163.Wang Y, Alam GN, Ning Y, Visioli F, Dong Z, Nor JE, Polverini PJ. The Unfolded Protein Response Induces the Angiogenic Switch in Human Tumor Cells through the PERK/ATF4 Pathway. Cancer Res. 2012;72(20):5396–5406. doi: 10.1158/0008-5472.can-12-0474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Gargalovic PS, Imura M, Zhang B, Gharavi NM, Clark MJ, Pagnon J, Yang WP, He A, Truong A, Patel S, et al. Identification of inflammatory gene modules based on variations of human endothelial cell responses to oxidized lipids. Proc Natl Acad Sci U S A. 2006;103(34):12741–12746. doi: 10.1073/pnas.0605457103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Fallah A, Sadeghinia A, Kahroba H, Samadi A, Heidari HR, Bradaran B, Zeinali S, Molavi O. Therapeutic targeting of angiogenesis molecular pathways in angiogenesis-dependent diseases. Biomed Pharmacother. 2019;110:775–785. doi: 10.1016/j.biopha.2018.12.022. [DOI] [PubMed] [Google Scholar]
- 166.Galluzzi L, Baehrecke EH, Ballabio A, Boya P, Bravo-San Pedro JM, Cecconi F, Choi AM, Chu CT, Codogno P, Colombo MI, et al. Molecular definitions of autophagy and related processes. EMBO J. 2017;36(13):1811–1836. doi: 10.15252/embj.201796697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Rouschop KM, van den Beucken T, Dubois L, Niessen H, Bussink J, Savelkouls K, Keulers T, Mujcic H, Landuyt W, Voncken JW, et al. The unfolded protein response protects human tumor cells during hypoxia through regulation of the autophagy genes MAP1LC3B and ATG5. J Clin Invest. 2010;120(1):127–141. doi: 10.1172/jci40027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Yang X, Pan X, Zhao X, Luo J, Xu M, Bai D, Hu Y, Liu X, Yu Q, Gao D. Autophagy and Age-Related Eye Diseases. BioMed Res Int. 2019;2019:1–12. doi: 10.1155/2019/5763658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Claessen JH, Kundrat L, Ploegh HL. Protein quality control in the ER: balancing the ubiquitin checkbook. Trends Cell Biol. 2012;22(1):22–32. doi: 10.1016/j.tcb.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Obeng EA, Carlson LM, Gutman DM, Harrington WJ Jr., Lee KP, Boise LH. Proteasome inhibitors induce a terminal unfolded protein response in multiple myeloma cells. Blood. 2006;107(12):4907–4916. doi: 10.1182/blood-2005-08-3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Chen F-T, Yang C-M, Yang C-H. The Protective Effects of the Proteasome Inhibitor Bortezomib (Velcade) on Ischemia-Reperfusion Injury in the Rat Retina. PLoS ONE. 2013;8(5):e64262. doi: 10.1371/journal.pone.0064262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Hsu S-M, Yang C-H, Shen F-H, Chen S-H, Lin C-J, Shieh C-C. Proteasome Inhibitor Bortezomib Suppresses Nuclear Factor-Kappa B Activation and Ameliorates Eye Inflammation in Experimental Autoimmune Uveitis. Mediators Inflamm. 2015;2015:1–10. doi: 10.1155/2015/847373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Cornejo VH, Pihan P, Vidal RL, Hetz C. Role of the unfolded protein response in organ physiology: lessons from mouse models. IUBMB Life. 2013;65(12):962–975. doi: 10.1002/iub.1224. [DOI] [PubMed] [Google Scholar]
- 174.Wang W, Lian N, Li L, Moss HE, Wang W, Perrien DS, Elefteriou F, Yang X. Atf4 regulates chondrocyte proliferation and differentiation during endochondral ossification by activating Ihh transcription. Development. 2009;136(24):4143–4153. doi: 10.1242/dev.043281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Valenzuela V, Collyer E, Armentano D, Parsons GB, Court FA, Hetz C. Activation of the unfolded protein response enhances motor recovery after spinal cord injury. Cell Death Dis. 2012;3:e272. doi: 10.1038/cddis.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Hettmann T, Barton K, Leiden JM. Microphthalmia due to p53-mediated apoptosis of anterior lens epithelial cells in mice lacking the CREB-2 transcription factor. Dev Biol. 2000;222(1):110–123. doi: 10.1006/dbio.2000.9699. [DOI] [PubMed] [Google Scholar]
- 177.Iida K, Li Y, McGrath BC, Frank A, Cavener DR. PERK eIF2 alpha kinase is required to regulate the viability of the exocrine pancreas in mice. BMC Cell Biol. 2007;8:38. doi: 10.1186/1471-2121-8-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Zhang P, McGrath BC, Reinert J, Olsen DS, Lei L, Gill S, Wek SA, Vattem KM, Wek RC, Kimball SR, et al. The GCN2 eIF2α Kinase Is Required for Adaptation to Amino Acid Deprivation in Mice. Mol Cell Bio. 2002;22(19):6681–6688. doi: 10.1128/mcb.22.19.6681-6688.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Abraham N, Stojdl DF, Duncan PI, Méthot N, Ishii T, Dubé M, Vanderhyden BC, Atkins HL, Gray DA, McBurney MW, et al. Characterization of Transgenic Mice with Targeted Disruption of the Catalytic Domain of the Double-stranded RNA-dependent Protein Kinase, PKR. J Biol Chem. 1999;274(9):5953–5962. doi: 10.1074/jbc.274.9.5953. [DOI] [PubMed] [Google Scholar]
- 180.Harding HP, Zeng H, Zhang Y, Jungries R, Chung P, Plesken H, Sabatini DD, Ron D. Diabetes mellitus and exocrine pancreatic dysfunction in perk−/− mice reveals a role for translational control in secretory cell survival. Mol Cell. 2001;7(6):1153–1163. doi: 10.1016/s1097-2765(01)00264-7. [DOI] [PubMed] [Google Scholar]
- 181.Zhang P, McGrath B, Li S, Frank A, Zambito F, Reinert J, Gannon M, Ma K, McNaughton K, Cavener DR. The PERK eukaryotic initiation factor 2 alpha kinase is required for the development of the skeletal system, postnatal growth, and the function and viability of the pancreas. Mol Cell Biol. 2002;22(11):3864–3874. doi: 10.1128/mcb.22.11.3864-3874.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Wei J, Sheng X, Feng D, McGrath B, Cavener DR. PERK is essential for neonatal skeletal development to regulate osteoblast proliferation and differentiation. J Cell Physiol. 2008;217(3):693–707. doi: 10.1002/jcp.21543. [DOI] [PubMed] [Google Scholar]
- 183.Saito A, Ochiai K, Kondo S, Tsumagari K, Murakami T, Cavener DR, Imaizumi K. Endoplasmic reticulum stress response mediated by the PERK-eIF2(alpha)-ATF4 pathway is involved in osteoblast differentiation induced by BMP2. J Biol Chem. 2011;286(6):4809–4818. doi: 10.1074/jbc.M110.152900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Trinh MA, Kaphzan H, Wek RC, Pierre P, Cavener DR, Klann E. Brain-specific disruption of the eIF2alpha kinase PERK decreases ATF4 expression and impairs behavioral flexibility. Cell Rep. 2012;1(6):676–688. doi: 10.1016/j.celrep.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Jousse C, Oyadomari S, Novoa I, Lu P, Zhang Y, Harding HP, Ron D. Inhibition of a constitutive translation initiation factor 2α phosphatase, CReP, promotes survival of stressed cells. J Cell Biol. 2003;163(4):767–775. doi: 10.1083/jcb.200308075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Harding HP, Zhang Y, Scheuner D, Chen J-J, Kaufman RJ, Ron D. Ppp1r15 gene knockout reveals an essential role for translation initiation factor 2 alpha (eIF2α) dephosphorylation in mammalian development. Proc Natl Acad Sci U S A. 2009;106(6):1832–1837. doi: 10.1073/pnas.0809632106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Dever TE. Gene-specific regulation by general translation factors. Cell. 2002;108(4):545–556. doi: 10.1016/s0092-8674(02)00642-6. [DOI] [PubMed] [Google Scholar]
- 188.Scheuner D, Song B, McEwen E, Liu C, Laybutt R, Gillespie P, Saunders T, Bonner-Weir S, Kaufman RJ. Translational control is required for the unfolded protein response and in vivo glucose homeostasis. Mol Cell. 2001;7(6):1165–1176. doi: 10.1016/s1097-2765(01)00265-9. [DOI] [PubMed] [Google Scholar]
- 189.Scheuner D, Vander Mierde D, Song B, Flamez D, Creemers JW, Tsukamoto K, Ribick M, Schuit FC, Kaufman RJ. Control of mRNA translation preserves endoplasmic reticulum function in beta cells and maintains glucose homeostasis. Nat Med. 2005;11(7):757–764. doi: 10.1038/nm1259. [DOI] [PubMed] [Google Scholar]
- 190.Back SH, Scheuner D, Han J, Song B, Ribick M, Wang J, Gildersleeve RD, Pennathur S, Kaufman RJ. Translation attenuation through eIF2alpha phosphorylation prevents oxidative stress and maintains the differentiated state in beta cells. Cell Metab. 2009;10(1):13–26. doi: 10.1016/j.cmet.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Endo M, Oyadomari S, Suga M, Mori M, Gotoh T. The ER stress pathway involving CHOP is activated in the lungs of LPS-treated mice. J Biochem. 2005;138(4):501–507. doi: 10.1093/jb/mvi143. [DOI] [PubMed] [Google Scholar]
- 192.Chen CM, Wu CT, Chiang CK, Liao BW, Liu SH. C/EBP homologous protein (CHOP) deficiency aggravates hippocampal cell apoptosis and impairs memory performance. PLoS One. 2012;7(7):e40801. doi: 10.1371/journal.pone.0040801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Tajiri S, Oyadomari S, Yano S, Morioka M, Gotoh T, Hamada JI, Ushio Y, Mori M. Ischemia-induced neuronal cell death is mediated by the endoplasmic reticulum stress pathway involving CHOP. Cell Death Differ. 2004;11(4):403–415. doi: 10.1038/sj.cdd.4401365. [DOI] [PubMed] [Google Scholar]
- 194.Gao Y, Sartori DJ, Li C, Yu QC, Kushner JA, Simon MC, Diehl JA. PERK is required in the adult pancreas and is essential for maintenance of glucose homeostasis. Mol Cell Biol. 2012;32(24):5129–5139. doi: 10.1128/mcb.01009-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.