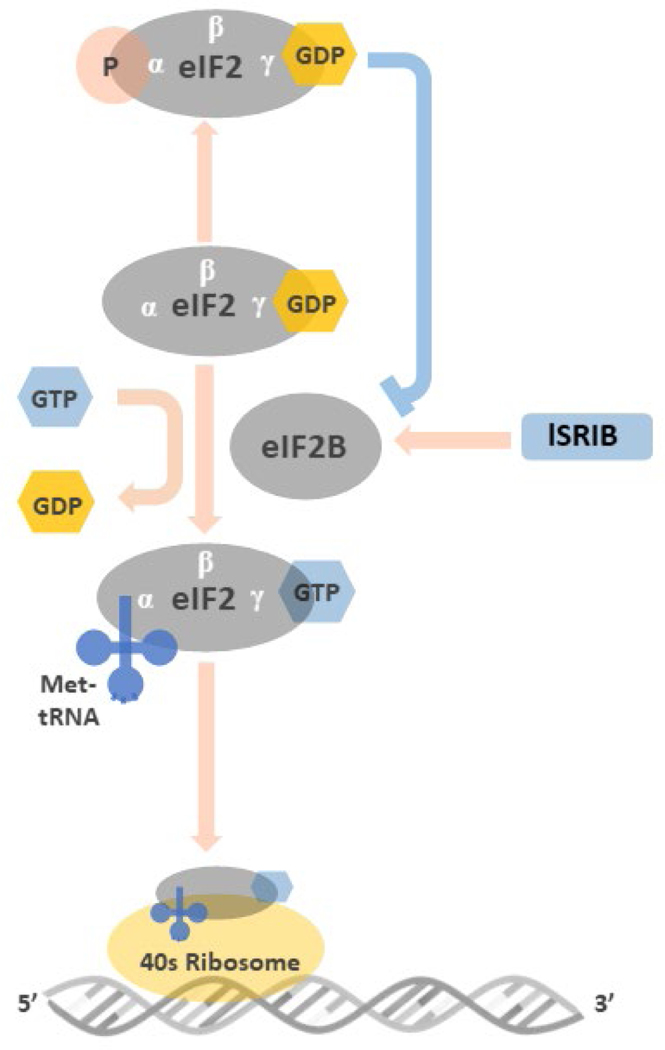
Figure 2: Illustration of the roles of eIF2 and peIF2 in the initiation of mRNA translation.
Before binding to met-tRNA, the GDP bound to the γ subunit of eIF2 must be switched to GTP. This GDP-GTP exchange process is mediated by eIF2B. The met-tRNA-GTP-eIF2 ternary complex then delivers the met-tRNA to 40S ribosome, a key step for the initiation of mRNA translation. At the stressed condition, once the ISR is activated and the GDP-eIF2 is phosphorylated in its α subunit, the structural change makes GDP-peIF2 no longer a suitable substrate of eIF2B but transforms to an inhibitor of eIF2B. The inhibition of eIF2B by GDP-peIF2 further reduces the pool of functional GTP-eIF2 to bind met-tRNA, and lowers the global protein synthesis via 5’cap-dependent mRNA translation. ISRIB rescues protein translation by stabilization and increasing eIF2B abundance that counteracts low level of GDP-peIF2α in treated cells.