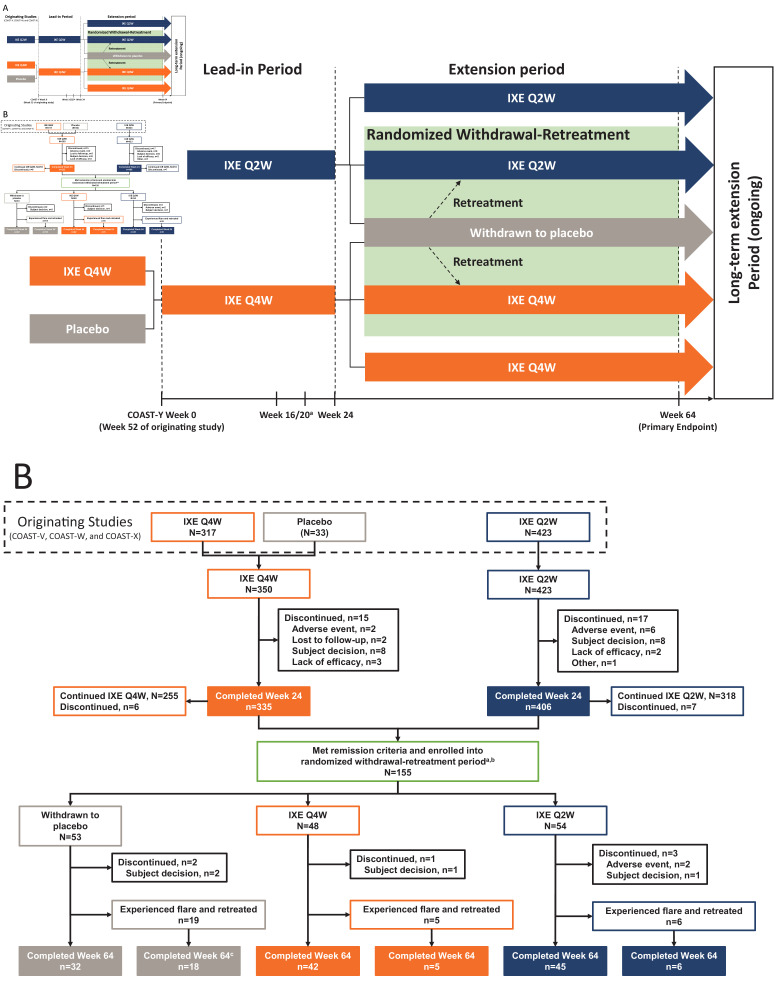
Figure 1.
COAST-Y study design (A) and patient flow diagram through week 64 of COAST-Y (B). Treatment groups from the originating studies indicate the assigned treatments at the final visit (week 52) of the originating studies. In addition, patient numbers from the originating studies include only those who entered the lead-in period of COAST-Y. The 33 patients receiving placebo at week 52 of the originating studies were from COAST-X. aPatients were eligible for entering the randomised withdrawal-retreatment period at week 24 if they achieved an Ankylosing Spondylitis Disease Activity Score (ASDAS) of <1.3 at least once during study visits at week 16 or week 20 and <2.1 at both visits. bA total of 157 patients met the remission criteria at week 20, but 2 patients discontinued prior to randomisation at week 24. cOne patient in the withdrawn to placebo group who experienced a flare and was retreated discontinued for reason of ‘subject decision’. IXE, ixekizumab; Q2W, every 2 weeks; Q4W, every 4 weeks.