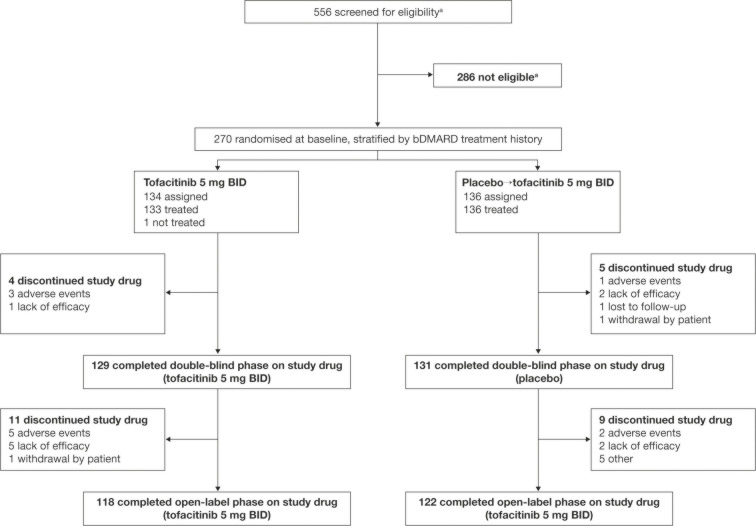
Figure 1.
Patient disposition. Data are from the week 48 final analysis. Patients receiving placebo in the double-blind phase advanced at week 16 to tofacitinib 5 mg two times per day for the open-label phase. aOne additional patient was screened and was considered to be not eligible; this patient did not provide any demographic data and is therefore not included in the formal patient disposition. bDMARD, biologic disease-modifying antirheumatic drug; BID, two times per day.