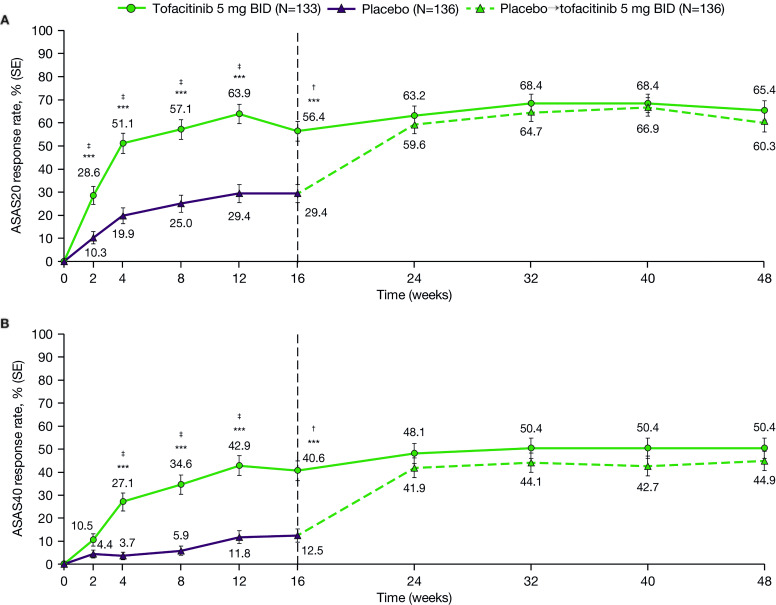
Figure 2.
Efficacy of tofacitinib 5 mg two times per day versus placebo→tofacitinib 5 mg two times per daya over time up to week 48: (A) ASAS20 responseb and (B) ASAS40 response.b Data up to week 16 are from the week 16 analysis: data cut-off 19 December 2019; data snapshot 29 January 2020. Data for weeks 24–48 are from the week 48 final analysis. ***p<0.001 for comparing tofacitinib 5 mg two times per day versus placebo. †p≤0.05 for comparing tofacitinib 5 mg two times per day versus placebo, according to the prespecified step-down testing procedure for global type I error control. ‡p≤0.05 for comparing tofacitinib 5 mg two times per day versus placebo, according to the prespecified step-down testing procedure for type I error control of ASAS response over time. aPatients receiving placebo advanced to tofacitinib 5 mg two times per day at week 16 (dashed line). bUp to week 16, response rate was tested in hierarchical sequence to control for type I error: weeks 16, 12, 8, 4 and 2. Statistical significance could be declared only if the prior time points in the sequence met the requirements for significance (p≤0.05). After week 16, there was no type I error control. Normal approximation adjusting for the stratification factor (bDMARD treatment history: bDMARD-naïve vs TNFi-IR or prior bDMARD use without IR) derived from the clinical database via the Cochran-Mantel-Haenszel approach was used. Missing response was considered as non-response. ASAS, Assessment of SpondyloArthritis international Society; ASAS20, ASAS ≥20% improvement; ASAS40, ≥40% improvement; bDMARD, biologic disease-modifying antirheumatic drug; BID, two times per day; IR, inadequate response or intolerance; N, number of patients in full analysis set; TNFi, tumour necrosis factor inhibitor.