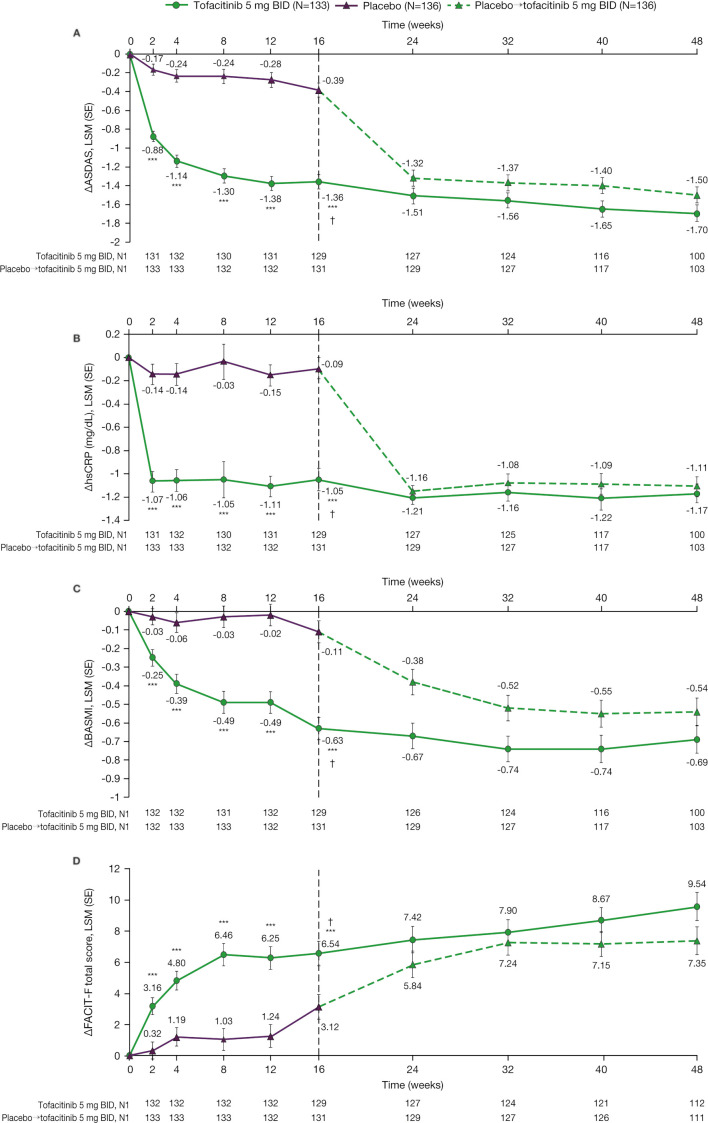
Figure 3.
Efficacy of tofacitinib 5 mg two times per day versus placebo→tofacitinib 5 mg two times per daya over time up to week 48: (A) ∆ASDAS,b (B) ∆hsCRP (mg/dL),b (C) ∆BASMIb and (D) ∆FACIT-F total score.b Data up to week 16 are from the week 16 analysis: data cut-off 19 December 2019; data snapshot 29 January 2020. Data for weeks 24–48 are from the week 48 final analysis. ***p<0.001 for comparing tofacitinib 5 mg two times per day versus placebo. †p≤0.05 for comparing tofacitinib 5 mg two times per day versus placebo, according to the prespecified step-down testing procedure for global type I error control. aPatients receiving placebo advanced to tofacitinib 5 mg two times per day at week 16 (dashed line). bMixed model for repeated measures included fixed effects of treatment group, visit, treatment group by visit interaction, stratification factor (bDMARD treatment history: bDMARD-naïve vs TNFi-IR or prior bDMARD use without IR) derived from the clinical database, stratification factor by visit interaction, baseline value, and baseline value by visit interaction. The model used a common unstructured variance–covariance matrix, without imputation for missing values. Two separate models were used. In the analyses of results through the first 16 weeks, the data cut-off of 19 December 2019 was used; the results through week 16 are from this model. In the analyses of the results through week 48 (including all post-baseline data through week 48), the week 48 final data were used; the results from week 24 through week 48 are from this model. ∆, change from baseline; ASDAS, Ankylosing Spondylitis Disease Activity Score using hsCRP; BASMI, Bath Ankylosing Spondylitis Metrology Index; bDMARD, biologic disease-modifying antirheumatic drug; BID, two times per day; FACIT-F, Functional Assessment of Chronic Illness Therapy-Fatigue; hsCRP, high-sensitivity C-reactive protein; IR, inadequate response or intolerance; LSM, least squares mean; N, number of patients in full analysis set; N1, number of patients with observation at visit; TNFi, tumour necrosis factor inhibitor.