Figure 6.
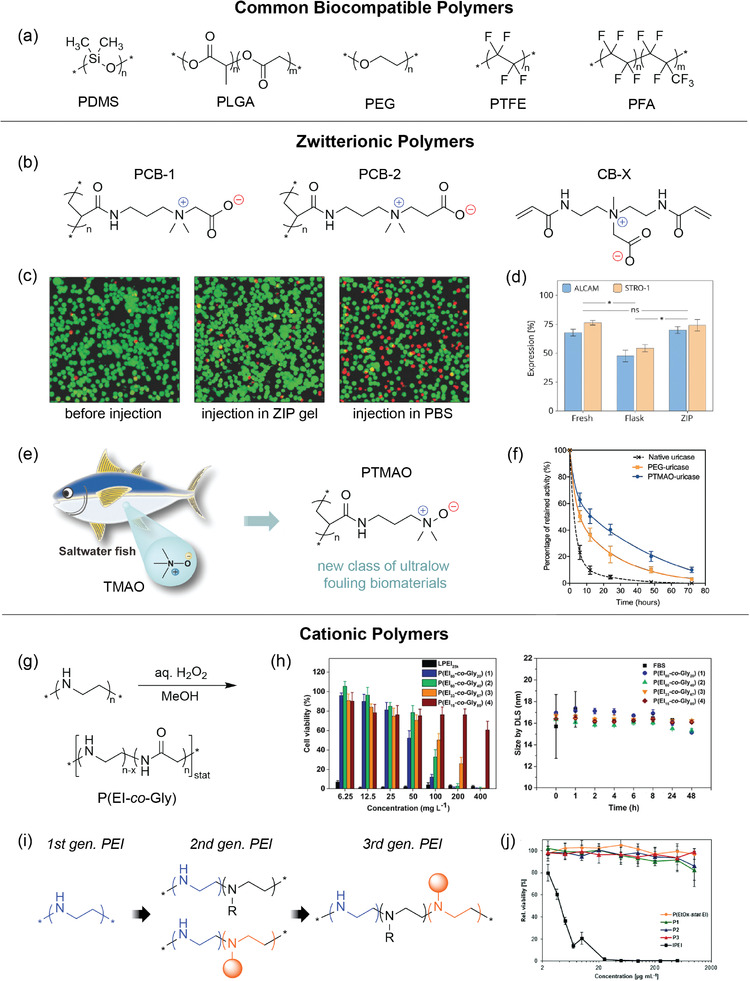
Molecular design for common and emerging biocompatible polymers. a) Chemical structures of biocompatible polymers commonly used in medical applications. b) Chemical structures of the injectable zwitterionic hydrogel platform based on carboxybetaine (CB) polymers and crosslinker. c) LIVE/DEAD stained HEK‐293T cells before and after injection in ZIP gel and PBS control as well as d) expression of multipotency biomarkers ALCAM and STRO‐1 after culture in control flasks and ZIP gels demonstrate improved biocompatibility of the hydrogel. Adapted with permission.[ 109 ] Copyright 2018, Wiley‐VCH. e) The design of PTMAO is derived from TMAO, a zwitterionic osmolyte in saltwater fishes. f) Pharmacokinetics profile of each uricase protein sample after the third intravenous (IV) injection were determined by measuring the retained activity in mice sera. Adapted with permission.[ 52 ] Copyright 2019, AAAS. g) Scheme of the oxidation of commercial linear PEI to P(EI‐co‐Gly) by H2O2. h) Cell viability and serum stability of linear PEI as well as P(EI‐co‐Gly) copolymers. Adapted with permission.[ 53 ] Copyright 2015, American Chemical Society. i) Different generations of linear PEI. The multifunctional third generation PEI outperforms first (PEI) and second (single PEI modifications) generations in terms of biocompatibility and biodegradability. j) Relative viability of L929 cells after 24 h incubation with PEI and PEI copolymers at different concentrations. Adapted with permission.[ 50 ] Copyright 2017, Royal Society of Chemistry.
