Figure 7.
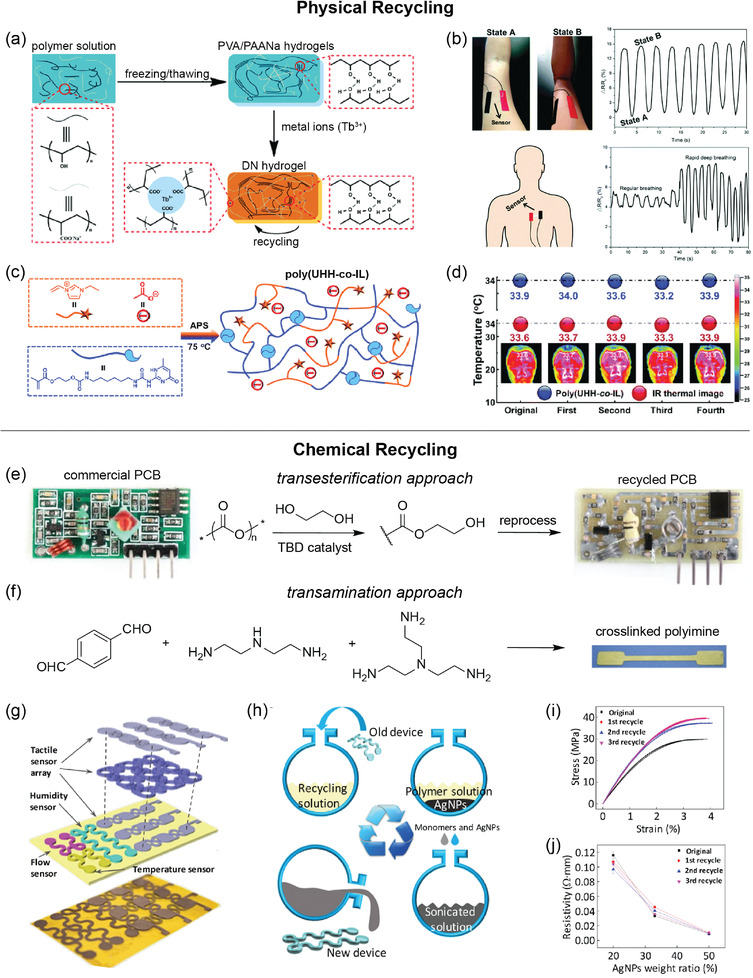
Physically and chemically recyclable polymer‐based electronics. a) Schematic illustration of the preparation and recycling of DN‐hydrogel network structures. The PVA/PAANa hydrogels were formed through three freezing/thawing cycles of polymer solution, and the addition of metal ions effectively enabled crosslinking between Tb3+ and its carboxyl groups. b) Photographs and the relative resistance changes of the wearable DN‐hydrogel strain sensor when the forearm was bent and unbent (top) as well as when regular breathing and rapid deep breathing were conducted (bottom). Adapted with permission.[ 24 ] Copyright 2018, Royal Society of Chemistry. c) Schematic illustration of the recyclable, conductive supramolecular polymer based on an ionic liquid crosslinked by quadruple hydrogen‐bonding interactions. APS: ammonium persulfate d) Forehead temperature measurements via a poly(UHH‐co‐IL) sensor recycled four times and IR thermography. Adapted with permission.[ 121 ] Copyright 2018, Royal Society of Chemistry. e) Small molecule‐assisted dissolution method using ethylene glycol and catalytic triazabicyclodecene (TBD) for the recycling of commercial printed circuit boards. Adapted with permission.[ 123 ] Copyright 2019, Springer Nature. f) Synthetic scheme for polymerization of the polyimine substrate. g) Schematic illustration (top) and optical image (bottom) of the e‐skin and its multiple sensors made with recyclable polyimine substrate. h) Schematic illustration of an old device soaked in recycling solution and decomposed into oligomers/monomers and silver nanoparticles (AgNPs). After recycling, the solution and AgNPs can be mixed to make new devices. i) Stress–strain curves and j) electrical resistivity measurements of the conductive polyimine films before and after recycling, displaying comparable mechanical properties and electrical performance. Adapted with permission.[ 23 ] Copyright 2018, AAAS.
