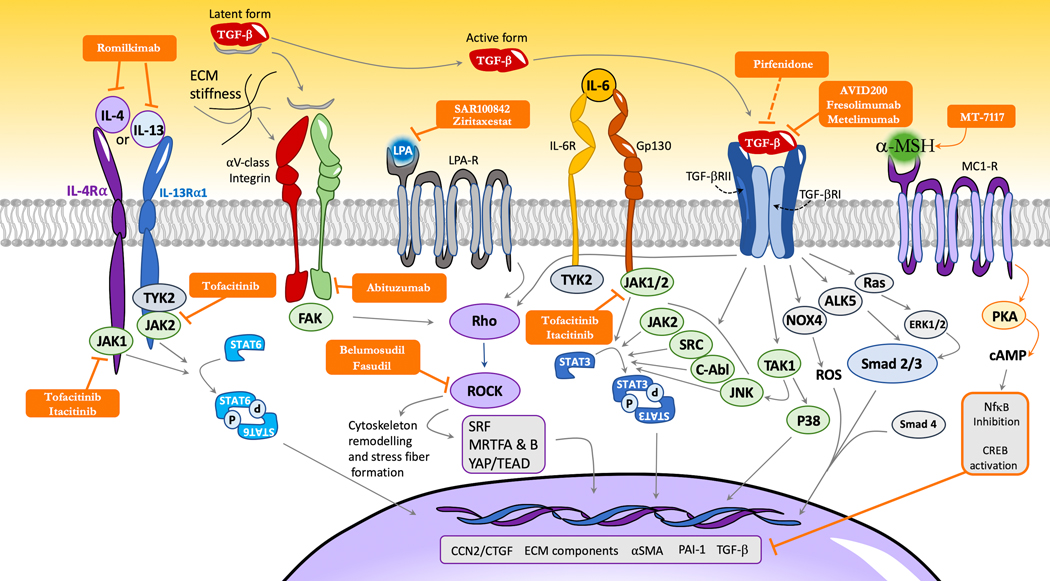
Figure 2: Non approved pharmacological targets and interaction of selected profibrotic pathways in SSc myofibroblasts that are currently being evaluated in clinical trials.
Latent TGF-β can notably be activated by integrins (notably from the αV class) or thrombospondins. In its active form, TGF-β can interact with a specific heterodimeric receptor (TGF-β-R-I and -II). Two TGF-β-dependent signalization pathways are described: a canonical pathway involving the interaction of TGF-β-R-I with smad 2/3 and 4; and a non-canonical pathway, Smad independent, that notably involves (but is not limited to) c-Abl, TAK1, p38, JNK, SRC, RhoA/ROCK and JAK2-STAT-3. TGF-β1 also increases SSc-related oxidative stress notably through the up-regulation of NADPH oxidase (NOX) 4. This upregulation of NOX4 by TGF-β1 is smad3 dependent and results in increased collagen type I, alpha-SMA and fibronectin 1 gene expression in dermal fibroblasts. These effects are suppressed by pan-NOX inhibitors such as diphenyleneidonium (not represented) or specific NOX1/4 inhibitor such as GKT-137831 (not represented), highlighting that NOX4 may constitute a relevant therapeutic target (152,186). IL-6 mediates its effects through its receptor (composed of IL-6Ra or the soluble form of IL-6R in association with a 130kDA signaling transducer (gp130)) that activates JAK1/2/TYK2 with subsequent phosphorylation of STAT3 (predominantly) and STAT 1. STAT-3 acts as a key integrator of profibrotic signals. STAT3 notably promotes the production of key extra-cellular matrix components such as col1a1, col1a2, and profibrotic markers such as CTGF/CCN2 (10). CTGF/CCN2 exerts profibrotic properties notably as a co-factor of TGF-β signaling and as a target of YAP. CTGF/CCN2 can interact with specific receptors (such as integrins or lipoprotein receptor-related proteins), extra-cellular matrix proteins (such as fibronectin or perlecan) and growth-factors (such as VEGF and TGF-β), with subsequent activation of fibroblast proliferation and myofibroblasts activation, although the specific mechanisms involved are still to be determined (184,185). Rho serves as another integrator of profibrotic pathways and can be activated by TGF-β, LPA agonists or integrin signaling in a FAK dependent manner. Rho subsequently activates ROCK that participates in a cascade sustaining fibrotic response and induces cytoskeleton remodeling. In return, increased extra-cellular matrix stiffness participates in the activation of latent TGF-β perpetuating a pro-fibrotic pathogenic loop. IL-4 and IL-13 participate in fibrosis by inducing the proliferation of fibroblasts and by increasing their production of TGF-β and CTGF/CCN2 in a STAT6 dependent manner (103). On the contrary, α-MSH and MC1-R agonists may exert anti-fibrotic properties by suppressing TGF-β signaling although the precise sub-cellular mechanisms are still to be determined.
FAK=focal adhesion kinase, LPA= lysophosphatidic acid; LPA-R=LPA-Receptor; IL-6R=IL-6 receptor; TGF-βRI & II= TGF-β receptor I & II; PAI-1=plasminogen activator inhibitor 1; ROS=Reactive Oxygen Species; YAP=Yes Associated Protein; α-MSH =α-Melanocyte-stimulating hormone; IL-4Rα=IL-4 receptor α; IL-13Rα1= IL-13 receptor α1; ECM=Extra-cellular matrix