Fig. 2.
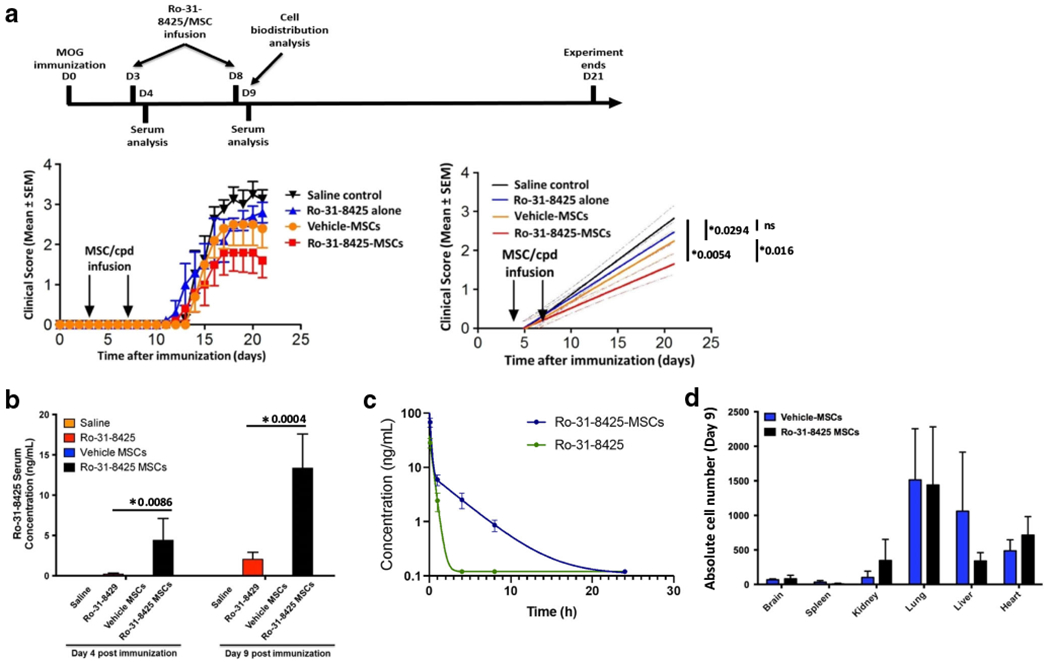
Ro-31-8425 pretreatment improves the therapeutic impact of prophylactic MSC administration in EAE, without altering the MSC global bio-distribution. C57Bl/6 mice were immunized with MOG35–55/CFA and developed remitting-relapsing EAE after 9-13 days. Ro-31-8425 (18.75 μg/kg, 375 ng/mouse) or MSCs (vehicle- or Ro-31-8425-pretreated, 0.25 × 106/mouse) were administered intravenously twice into each mouse on days 3 and 8 post immunization. a Clinical score (right), and linear-regression analysis (left). Clinical scores are mean ± SEM, and representative of two independent experiments, n = 5 mice per group.*p < 0.05, n.s. not significant, p values are indicated in the graph and were determined by two-way analysis of variance (ANOVA). b Twenty-four hours after the second infusion (day 9 post MOG immunization), blood samples were obtained, and Ro-31-8425 serum levels were assessed by LC-MS (*p < 0.05, p values are indicated in the graph, one-way ANOVA using Tukey’s HSD, error bars represent SD). c A single dose of Ro-31-8425 (18.75 μg/kg, 375 ng/mouse) or Ro-31-8425-pretreated MSCs (0.25 × 106/mouse) were administered intravenously into C57Bl/6 mice. The serum concentrations of Ro-31-8425 at indicated time points were quantified by LC-MS. d EAE b6 mice received the above described treatments of Ro-31-8425 alone, vehicle MSCs, or Ro-31-8425-MSC treatments (MSCs were fluorescently stained using violet CellTrace prior to each infusion). Twenty-four hours post the second infusion (day 9), mice were sacrificed, and MSC presence in the CNS (brain and spinal cord), kidneys, lungs, spleen, gut, and heart was assessed by flow cytometry (n = 3 mice per group)
