Figure 1.
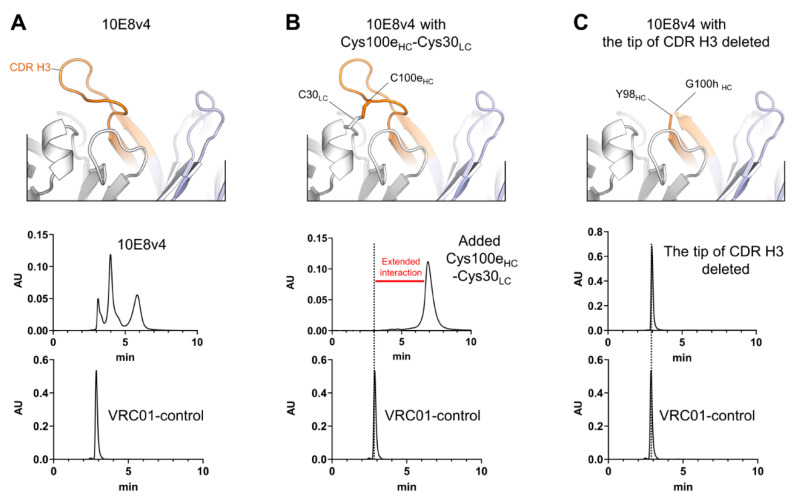
CDR H3 of 10E8 is the cause of extended interaction with SEC. (A) Top panel, crystal structure of 10E8v4 (PDB ID:5IQ9); bottom panels, anomalous SEC profile of 10E8v4 versus antibody VRC01 control. (B) After introducing a disulfide bond by mutation of CDR H3 (Tyr100eCys) and light chain (Ser30Cys), a single peak of the 10E8v4 modified Cys100eHC-Cys30LC was observed, but with anomalously longer SEC profile compared to antibody VRC01. Top panel, model of the disulfide bond linked CDR H3; bottom panels, SEC chromatography of 10E8 variant highlighting anomalous SEC interaction versus antibody VRC01 control. (C) Deletion of the tip of CDR H3 (99YDFWFGYPP100g) leads to loss of extended SEC interactions. Top panel, model of the 10E8v4 structure with the tip of CDR H3 deleted; bottom panels, SEC chromatography of 10E8 variant highlighting anomalous SEC interaction versus antibody VRC01 control.