Figure 7. Therapeutic intratracheal delivery of AAV1.hSIN3a reversed SuHx-induced PAH.
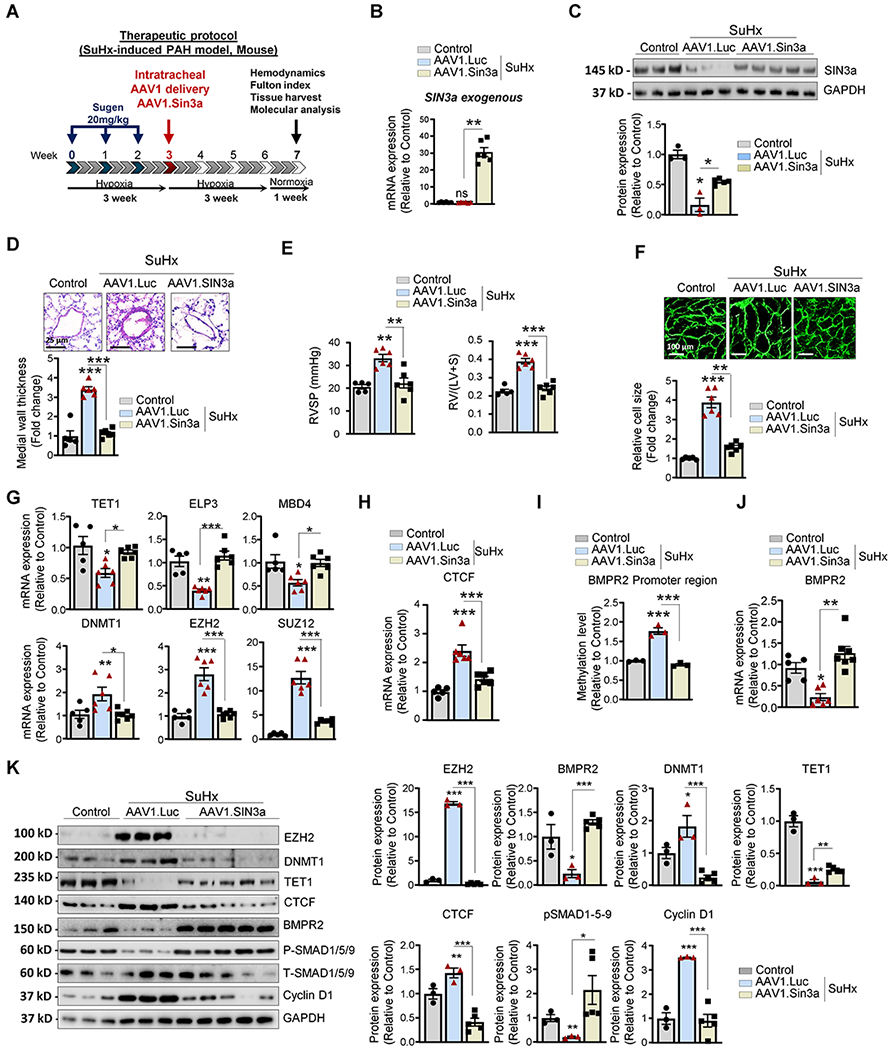
A. Schematic of the experimental design to assess the therapeutic efficacy of AAV1.hSIN3a gene therapy in the SuHx-induced PAH mouse model. Tissues were collected at week 7 for molecular and histology analysis. B. Exogenous SIN3a mRNA level was assessed in lung tissues by RT-qPCR in the mice lungs to determine the efficiency of the IT delivery of AAV1.hSIN3a gene transfer. C. SIN3a protein expression was assessed by immunoblotting. D. Representative hematoxylin and eosin-stained lung sections of the indicated mice. The graph represents the quantification of the medial thickness. E. RVSP (left) and Fulton’s index (right) were determined in control, and SuHx-induced PAH mice treated either with AAV1.CT or AAV1.hSIN3a. F. RV sections were stained with fluorescence-tagged wheat germ agglutinin to measure RV cardiomyocyte cross-sectional area. G. Expression of the transcripts TET1, ELP3, MBD4, DNMT1, EZH2, SUZ12 was measured by RT-qPCR in lungs from control, and SuHx-induced PAH mice treated either with AAV1.CT or AAV1.hSIN3a. H. mRNA expression of CTCF was measured by RT-qPCR. I. The methylation level of the BMPR2 promoter region was analyzed by MS-PCR in control, and SuHx-induced PAH mice treated either with AAV1.CT or AAV1.hSIN3a. J. BMPR2 mRNA expression was assessed in the indicated groups by RT-qPCR. K. Lung homogenates were analyzed by immunoblotting for the indicated proteins. Representative western blots and respective densitometric quantitation for EZH2, BMPR2, DNMT1, TET1, CTCF, pSMAD1/5/9, and Cyclin D1 are shown. Protein expression was normalized to Total-SMAD and GAPDH. Data are presented as mean ±SEM; ns=not significant, * = p<0.05, ** = p<0.01, *** P < 0.001.
