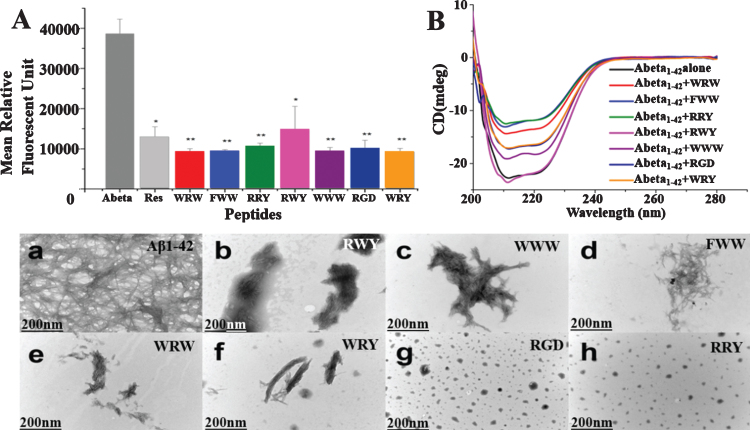
Fig. 3.
Small peptides inhibit Aβ1–42 aggregation in vitro. A) Inhibition of Aβ1–42 (2.5μM) aggregation by the 7 small peptides with resveratrol as a reference. The measurements were carried out by ThT fluorescence at 485 nm in presence of 2.5μM compounds including Resveratrol, WRW, FWW, RRY, RWY, WWW, RGD, and WRY. Most of the small peptides had higher inhibition of Aβ1–42 aggregation than the reference compound resveratrol. The mean relative fluorescence unit of Aβ1–42 and Aβ1–42 incubated with the compounds were statistically different. The values were the means±S.E.M (*p < 0.05; **p < 0.01, n = 6). B) CD spectroscopy showed less β-sheet structure formation signal in the peptide-Aβ complexes. Aβ1–42 (5μM) samples were incubated at 37°C for 48 h alone (black line) or in the presences of RWY (5μM) (light purple), WWW (5μM) (dark purple), RGD (5μM) (dark blue), WRY (5μM) (orange), WRW (5μM) (red), FWW (5μM) (light blue), or RRY (5μM) (green), respectively. In lower panel, small peptides inhibit the aggregation of Aβ1–42 under negative stains TEM. Samples were dissolved (25μM) and incubated at 37°C for 48 h. a) Aβ1–42 peptide alone. Long linear Aβ1–42 fibrils were shown. The fibrils compact in parallel bundles and intercross with each other, forming radial nucleation center, which was shown with arrowheads. b-h) Aβ1–42 treated with small peptides. Only a few short fibrils (b, c, d, e, f) or amorphous aggregates (g, h) are seen. Fibrils and amorphous were also shown with arrowheads. Scale bar = 200 nm.