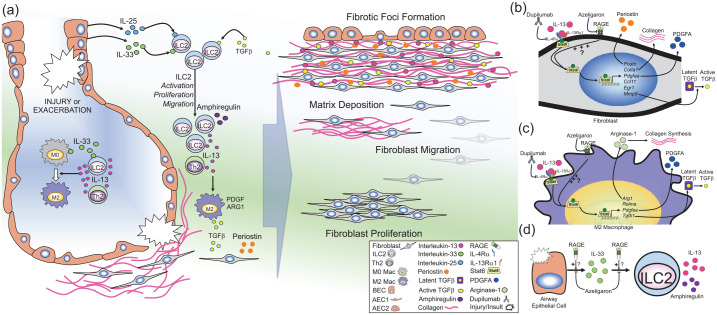
Figure 2.
RAGE-mediated regulation of type 2 immunity.
Illustration of the prospective role of RAGE in regulating type 2 inflammation in pulmonary fibrosis. (a) Lung injury or insult caused by known or unknown factors can promote exacerbations or disease progression in IPF. Injury in the airways can cause the release of IL-25 and IL-33 from lung epithelial cells [e.g. bronchial epithelial cells (BECs) or alveolar epithelial cells (AECs)]. IL-33 can also be produced by lung macrophages. IL-25, IL-33 and transforming growth-factor beta (TGFβ) induce the activation, proliferation, and migration of type 2 innate lymphoid cells (ILC2s). ILC2s [as well as T-helper 2 (Th2) cells] produce large amounts of IL-13, while ILC2s also produce amphiregulin. IL-13 promotes the polarization of naïve macrophages (M0) to profibrotic (M2) macrophages which produce TGFβ, platelet-derived growth factor (PDGF), and arginase-1 (ARG1). In fibroblasts (or activated myofibroblasts), IL-13 stimulates production of periostin and TGFβ and promotes fibroblast activation. Together, the molecules promote fibroblast proliferation, migration, and matrix deposition, which eventually leads to the development of fibrotic foci in the interstitial space near regenerative airways. (b–d) Potential cellular mechanisms of RAGE signaling in development of fibrosis: (b) In fibroblasts, IL-13 binds to its cognate receptor complex which consists of IL-4Rα and IL-13Rα1, which induces phosphorylation (P) of Stat6, inducing nuclear translocation and subsequent expression of Stat6-target genes [e.g. periostin (Postn), collagen type 1A1 (Col1a1), PDGF-AA (Pdfgaa), chemokine C–C motif containing ligand-11 (Ccl11), early growth response-1 (Egr1), and matrix metalloproteinase-9 (Mmp9). This leads to production of periostin, collagen, PDGFA and MMP-9, which in turn can activate latent TGFβ. (c) In macrophages, stimulation with IL-13 causes polarization to the M2 phenotype, leading to Stat6-dependent expression of ARG-1 (Arg1) which promotes collagen production, resistin-like molecule alpha (Relma), Pdgfaa, and TGFβ-1 (Tgfb1). RAGE promotes IL-13/Stat6 signaling in airway epithelial cells. (b–c) Therefore, it is hypothesized that this RAGE-mediated mechanism may also contribute to IL-13/Stat6 signaling in fibroblasts and macrophages, promoting fibrogenesis in IPF, which could be inhibited by RAGE-specific small-molecule antagonist (azeligaron) or the monoclonal anti-IL-4Rα antibody (dupilumab). Use of such treatments have the potential to reduce disease progression in IPF. (d) Damage to airway epithelial cells can induce the release of IL-33, which in turn promotes the proliferation, migration, and activation of ILC2s, which in turn produce large amounts of IL-13 and amphiregulin. In models of asthma, RAGE promotes accumulation of IL-33 in the airways in response to allergens, and furthermore, RAGE also promotes ILC2 activation and accumulation in response to exogenous IL-33. Therefore, panel (d) suggests that RAGE may also promote IL-33/ILC2-signaling in IPF, which could be inhibited by RAGE-specific small-molecule antagonists (e.g. azeligaron).
IL, interleukin; IPF, idiopathic pulmonary fibrosis; RAGE, receptor for advanced glycation endproducts; Rα, receptor alpha; Stat6, signal transducer and activator of transcription 6.