Figure 3.
The calprotectin–RAGE axis in pulmonary fibrosis.
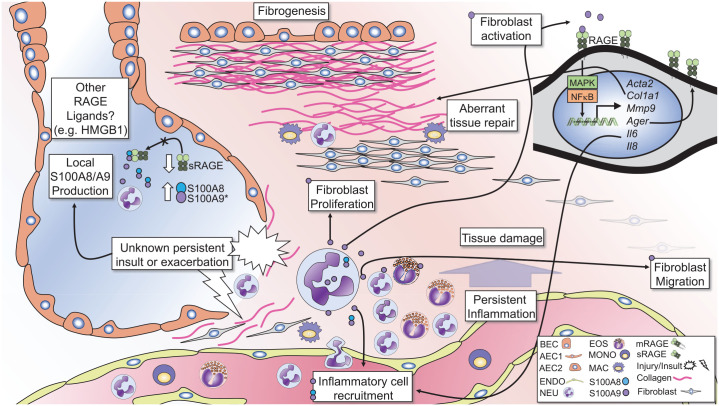
The diagram illustrates the potential role of S100A8/A9-RAGE signaling in inflammation and fibrogenesis in IPF. S100A8 and S100A9 are elevated in the lungs of subjects with pulmonary fibrosis and correlates with lung neutrophil (NEU) and eosinophil (EOS) numbers. Conversely, sRAGE levels are reduced in the lungs of subjects with IPF, decreasing the ability of sRAGE to inhibit S100A8/A9 signaling. Neutrophils are the primary source of S100A8/A9 and promote granulocytic inflammation. Persistent injury or exacerbations can lead to damage of alveolar epithelial cells (AECs), bronchial epithelial cells (BECs) and the surrounding parenchyma. These exacerbations lead to persistent inflammation causing damage to the lung architecture and subsequent aberrant tissue repair mechanisms, promoting fibrogenesis. S100A8/A9 and S100A9 alone can signal through RAGE to induce fibroblast migration, proliferation, and activation. In fibroblasts, S100A9 signals through RAGE to activate MAPK and NFkB pathways and induces expression of alpha smooth muscle actin (Acta2), Collagen 1A1 (Col1a1), matrix metalloproteinase-9 (Mmp9), and RAGE (Ager). S100A9 also induces expression of the pro-inflammatory cytokines IL-6 and IL-8, which promote inflammatory cell recruitment. Such signaling mechanisms may accompany persistent damage and concurrent inflammation, leading to proliferation and recruitment of fibroblasts to areas of tissue damage, promoting fibrosis in the lungs.
HMGB1, high mobility group box-1; IL, interleukin; IPF, idiopathic pulmonary fibrosis; RAGE, receptor for advanced glycation endproducts; sRAGE, soluble-form RAGE.