Table 2.
Structures of reference inhibitors and top docked phytochemicals with the active sites of 3CLpro of Coronaviruses.
| S/N | Compounds | Class of compounds | Chemical Structure | Source Plants |
|---|---|---|---|---|
| S1 | Lopinavir | 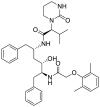 |
||
| S2 | Ritonavir | 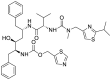 |
||
| S3 | R30 | 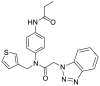 |
||
| 1 | Vernolide | Sesquiterpene lactones | 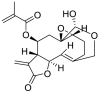 |
Vernonia amygdalina |
| 2 | Vernomygdin | Sesquiterpene lactones | 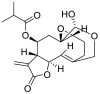 |
Vernonia amygdalina |
| 3 | 11, 13-dihydrovernodalin | Sesquiterpene lactones | 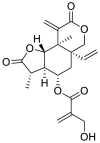 |
Vernonia amygdalina |
| 4 | Chicoric acid | Phenolic acids | 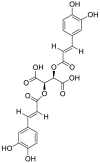 |
Occinum gratissimum |
| 5 | Rosmarinic acid | Phenolic acids | 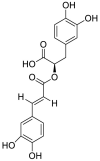 |
Occinum gratissimum |
| 6 | Luteolin | Flavonoids | 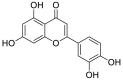 |
Occinum gratissimum |
| 7 | Neoandrographolide | Diterpenoid lactone | 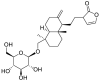 |
Vernonia amygdalina |
| 8 | Vernomenin | Sesquiterpene | 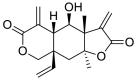 |
Vernonia amygdalina |
| 9 | myricetin | Flavonoids | 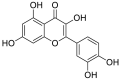 |
Occinum gratissimum |
| 10 | Isorhamnetin | Flavonoids | 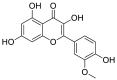 |
Vernonia amygdalina |
