Abstract
Astrocytes are dynamic cells residing in the central nervous system exhibiting many diverse functions. Astrocytes quickly change and present unique phenotypes in response to injury or disease. Here, we briefly summarize recent information regarding astrocyte morphology and function and provide brief insight into their phenotypic changes following injury or disease. We also present the utility of in vitro astrocyte cultures and present recent advances in biomaterial development that enable better recapitulation of their in vivo behavior and morphology.
Astrocytes: Morphology, function, and heterogeneity
Astrocytes are star-shaped glial cells which constitute about 50% of all the cells in the central nervous system (CNS) and play a vital role in maintaining CNS homeostasis [1]. They are derived from the radial glia in the neuroepithelium [2], which differentiate into mature astrocytes after neurogenesis and translocate to their destination in the white or gray matter [3–6]. They lose their bipolar morphology and form additional processes while upregulating their expression of the glial fibrillary acidic protein (GFAP). Astrocytes can dramatically alter their shape in the cortex and spinal cord (ex. in response to an injury) unlike the neurons and oligodendrocytes, which take on distinct morphology after terminal differentiation.
Astrocytes have long been characterized into 2 classes (Figure 1), primarily based on their morphology [7]. They, however, also differ in their developmental origin, location and antigenic phenotype [8]. Protoplasmic astrocytes have short and extensively branched processes with a bushy architecture and are predominantly found in the gray matter. They are arranged in non-overlapping domains and envelop neuronal cell bodies and synapses and weakly express GFAP [9]. Fibrous astrocytes, on the contrary, express GFAP, have long and elaborate processes and are found in the white matter where they interact with the nodes of Ranvier [10,11]. Albeit this classification, it has been shown that astrocytes demonstrate significantly variable genetic and molecular expression profiles depending on the different brain regions they are isolated from. Some notable mentions include Müller cells and Bergmann glial cells [12]. This inherent diversity of astrocytes may have important implications for their functions and response to insult or disease.
Figure 1:
Left: Based on their morphology, astrocytes found in the central nervous system are characterized into two prominent classes: protoplasmic and fibrous. Right: Following an insult, astrocytes turn reactive and show a change in their phenotype. However, the nature of response varies depending on the nature of the insult.
The functional diversity of astrocytes is also influenced by their state of maturity. Immature astrocytes have been shown to provide a favorable substrate for axonal growth by interacting with neuronal growth cones during development [13–15]. Mature astrocytes, however, do not have the same ability to promote neurite outgrowth. Injury to the CNS in adult mammals causes mature astrocytes to become reactive and lead to the formation of a glial scar that surrounds the damaged tissue [16]. Such injuries in immature mammals or lower species, however, result in a more reparative type of reactive astrogliosis, suggesting that there is an innate difference between immature and mature glia and that astrocytes change their properties as they mature.
Research over the past few decades has shown that astrocytes play a very crucial role in the CNS and not just act as passive support cells for neurons. They are important in maintaining tissue homeostasis by regulating intracellular/extracellular ion and neurotransmitter concentrations [17]. They regulate inflammatory factors, such as cytokines, chemokines, complement proteins and reactive oxygen species or reactive nitrogen species [18]. They are responsible for the formation and maintenance of the blood brain barrier and controlling the local blood flow [19,20]. They facilitate synaptogenesis and are involved in synaptic plasticity and pruning. They also play a vital role in providing structural, metabolic and trophic support for neurons [21–24].
Reactive Astrogliosis: Astrocytic response to CNS injury
An injury to the CNS typically results in a cascade of cellular and molecular changes, the magnitude of which depends on the severity of the injury. Astrocytes, in particular, undergo a dramatic change in their gene expression, proliferation, morphology and physiology, a process collectively known as reactive astrogliosis [25]. The injury transforms native astrocytes into reactive astrocytes, which eventually become scar-forming astrocytes (Figure 1) [26]. The astrocytes in the immediate vicinity of the injury become hypertrophic and show elongated morphologies with overlapping domains (most of which are fibrous astrocytes), while the astrocytes more distant from the lesion maintain their stellate morphology and non-overlapping domains suggesting a spatial heterogeneity in this process [27]. These morphological changes are accompanied by a modest increase in the proliferation of astrocytes along with an increase in their production of several key proteins such as GFAP and vimentin, markers widely used to identify reactive astrocytes, as well as S100β and nestin [28–33]. They also release neurotoxic molecules such as lipocalin. They upregulate their expression of proteoglycans, a range of inhibitory extracellular matrix proteins, prominent of which being aggrecan, brevican, neurocan, NG2, phosphacan, and versican [34–36]. These proteoglycans form an inhibitory gradient known as a glial scar that is highest at the lesion center and diminishes gradually into the penumbra, and houses reactive astrocytes, microglia, infiltrating macrophages, and endothelial cells [37,38]. The glial scar contains and prevents the spread of the damage to the surrounding healthy tissue, while also acting as a physical barricade to the regenerating axons and scar formation is driven by type 1 collagen via the integrin-N-cadherin pathway [26].
Gene transcriptome analysis of reactive astrocytes has shown that they exist in two distinct reactive states (Figure 1) –neurotoxic A1 astrocytes induced by neural inflammation and neuroprotective A2 astrocytes induced by ischemia [39,40]. A1 reactive astrocytes upregulate many classical complement cascade genes that are destructive to synapses, and secret neurotoxins while A2 reactive astrocytes can upregulate many neurotrophic factors, which can promote either the survival and growth of neurons or synaptic repair [41]. However, the signaling pathways driving these distinct states remain to be elucidated in the context of different CNS injuries.
In vitro Astrocyte Culture
Primary astrocyte cultures are generated to provide insight into astrocyte response to potentially beneficial pharmaceuticals, to chemokines expressed following injury or during disease, or to understand astrocyte interactions with other cells in the CNS. From a biomaterial perspective, primary astrocyte cultures provide useful information concerning the potential biocompatibility of new biomaterial designs that interface with the brain or spinal cord. Further, biomaterials can be used to create in vitro cues that recapitulate aspects of the in vivo environment. Before in vitro culture with biomaterials can occur, different perspectives of astrocyte culture should be considered.
Astrocytes used for culture are isolated from newborn mice or rats isolated from the brain or spinal cord [42]. However, location of tissue isolation is important, where white matter astrocytes are morphologically and phenotypically different than gray matter astrocytes and astrocyte physiology is distinct within specific anatomical regions as highlighted in the sections above [43]. Further, immature astrocytes from young animals continue to replicate in culture and are matured through the addition of fetal bovine serum (FBS) [44]. Culture protocols vary (medium composition, culture plate coating, and numbers of days in culture) and such variability can influence the extent of astrocyte reactivity [45]. More recently, protocols for creating human induced pluripotent stem cells (iPSCs) differentiated towards astrocytes are used to generate human astrocyte cultures [46]. Thus, human astrocyte cultures may supplant rodent astrocyte cultures as these protocols become more widely used.
The standard process to isolate and culture astrocytes typically produces astrocytes that are reactive in terms of their phenotype. Thus, alternative approaches may be used to isolate quiescent or naïve astrocytes that better represent uninjured or normal physiology. Also, the culture of astrocytes from young animals takes weeks to generate mature astrocyte cultures. To circumvent these challenges, astrocytes can be directly isolated from adult animals and separated from other cells using fluorescence-activated cell sorting (FACS) [47] or immunopanning [48].
In the following sections, we will present biomaterial platforms used to generate astrocyte cultures that better recapitulate in vivo astrocyte physiology and structure. Our focus highlights platforms that provide structures (topography) or mechanical properties or extracellular matrix (ECM; hydrogels) that better recapitulate the in vivo environment.
Engineering astrocyte morphology and function through topography
It has long been recognized that there is astrocyte morphological heterogeneity in the CNS, and, more recently, these morphological differences have begun to be studied to elucidate their impact on astrocyte functional heterogeneity [49]. Morphological and functional differences have proven difficult to study in vitro, where traditional two-dimensional astrocyte cultures more closely resemble reactive astrocytes than the astrocyte morphologies and phenotypes found in the healthy CNS [39]. The absence of three-dimensional architecture for astrocyte growth, limited extracellular matrix complexity, and a lack of cellular diversity found in typical CNS tissue all lead to the limited astrocyte heterogeneity found in culture. One engineering approach that researchers can employ to increase astrocyte heterogeneity in vitro is through the development of culture substrates that have nano and micro-topographical structures similar in size to those found in vivo.
The most common fabrication techniques for creation of three-dimensional topographical structures for in vitro astrocyte cultures have been polymer fiber electrospinning and photolithography. Topographic features must be in the size range that cells are able to sense and not bigger than the cells themselves, which has generally been shown to vary in the range of 100 μm to roughly 1 nm [50]. Astrocytes cultured on randomly oriented substrates in the range of 10μm –100nm take on a stellate morphology and extend processes (Figure 2) more closely resembling protoplasmic astrocytes compared to those cultured on two-dimensional substrates [51,52]. Many studies have shown that astrocytes cultured on uniaxially aligned topographies undertake a bipolar or stellate morphology oriented in the direction of the surface topography [53–56]. While our understanding of astrocyte functional changes associated with these observed morphological differences is still in its nascent stage, several interesting findings have begun to emerge. Astrocytes cultured on either uniaxially or randomly oriented micro- and nanotopographies show a decrease in GFAP expression that is typically associated with less reactive astrocyte phenotypes [52,53,56–58]. Glutamate transport has also been shown to be impacted, where increases in astrocyte glutamate transporter expression lead to increased astrocyte glutamate uptake and a neuroprotective effect on co-cultured neurons [55,56,59]. While these studies identify functional and morphological changes in astrocytes cultured on micro- and nanotopography, much more work is needed in this field to build a greater understanding of the impact of topography on astrocyte phenotype.
Figure 2:
Biomaterial topographies induce cultured astrocytes to become stellate and increase their expression of glutamate transporters while reducing their expression of GFAP.
To gain a deeper understanding into the biology of astrocytes cultured on micro- and nanotopographies, new technologies that are becoming available both in advanced material synthesis and cellular genotyping and phenotyping should be exploited. These technologies, including single-cell RNA sequencing, can be used to reveal astrocyte heterogeneity in culture, and will guide the design of topographies that more closely model astrocyte phenotypes found in vivo [60]. In the area of materials fabrication, micro- and nanotopographies that release therapeutics for extended time periods, display specific extracellular matrix molecules on their surfaces, and/or have distinct mechanical properties are being developed [61–63]. These technologies will introduce more precision and complexity into the astrocyte culture environment, allowing researchers greater control over the phenotypes they are able to produce in vitro. As researchers combine innovative technologies that precisely engineer culture environments with state-of-the-art analyses that determine astrocyte function, the ability to more closely mimic in vivo astrocyte phenotypes will begin to be realized.
Use of hydrogels within preclinical CNS injury models instructing their development for in vitro astrocyte culture
Hydrogels are appropriate materials for CNS injury since many are injectable and fill irregular geometries created by injury. Hydrogels are soft and many hydrogels nearly match the mechanical properties of the CNS, as mechanical mismatch between an implant and CNS tissue can spur further damage [64]. Hydrogels, at the lesion edge, create an interface to enable astrocyte attachment and infiltration. Thus, hydrogel placement within preclinical models have instructed the design of hydrogels for in vitro astrocyte culture.
Some hydrogels within in vivo settings are designed to facilitate moderate astrocyte attachment so that dense astrocyte networks or scarring is limited at the lesion edge. They may also limit interfacing between infiltrating blood monocytes and macrophages and astrocytes, which can drive astrocytes towards a reactive phenotype. Initial hydrogel designs implanted within spinal cord injury (SCI) models were composed of fibrin (with slowly releasing neurotrophin-3) [65] and subsequent designs consisted of hyaluronic acid [66], both of which were able to reduce GFAP expression following injury. More recently, polypeptide hydrogels releasing epidermal growth factor (EGF) and fibroblast growth factor (FGF) modified astrocyte behavior following SCI to stimulate robust axonal regeneration through astrocytic scars [67].
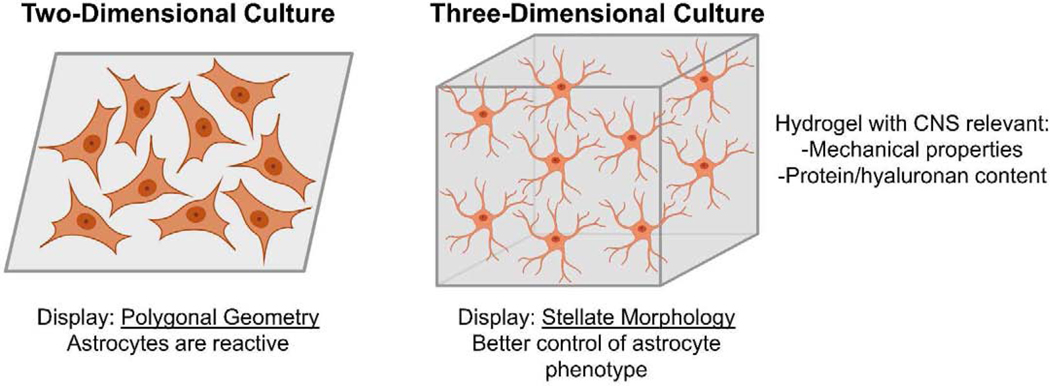
Results gathered from in vivo application have driven the design of in vitro hydrogels for astrocytes. Most recently, hydrogels for astrocyte culture and study are protein-based with doped hyaluronan; two such studies are highlighted here (Figure 3). A peptide hydrogel modeled after brain extracellular matrix was engineered to control astrocyte quiescence and activation as well as morphology [68]. This result is noteworthy since astrocyte morphology within hydrogels is often not stellate. Thus, recapitulating astrocyte shape observed in vivo may lead to cultures that better mimic astrocyte physiology. In another recent study, placement of hyaluronan into collagen hydrogels increased GFAP expression and increased astrocyte contractility [69]. Deciphering the roles of specific extracellular matrix molecules on astrocyte morphology and physiology will likely lead to more relevant hydrogel models for studying astrocyte behavior.
Figure 3:
Astrocytes on traditional two-dimensional surfaces display polygonal geometries and astrocytes on these surfaces are typically reactive. Three-dimensional hydrogels with distinct characteristics are able generate astrocytes displaying stellate forms and enable better control over astrocyte phenotype.
Biological assays to assess astrocytes
Considering the inherent heterogeneity of astrocytes observed in vivo, all the in vitro assays should consider the following aspects to enhance the translational potential of the assays: 1) source of the astrocytes, 2) activation state of the astrocytes and 3) appropriate readout from the astrocytes. As highlighted in the introduction, astrocytic structure and function depends heavily on where the astrocytes are isolated from and what their level of maturity is. So, the source of astrocytes is extremely important depending on the assay. For example, usage of mature astrocytes from the spinal cord would make for a better model to study spinal cord injury as opposed to immature astrocytes isolated from the cortex. Also, since the astrocytes are not in their naïve un-activated state following an insult, it is imperative that the assay is conducted using the appropriate stimulus to activate the astrocytes. For example, using a combination of interleukin 1 alpha (IL-1α), tumor necrosis factor alpha (TNFα), and complement component 1, q subcomponent (C1q) to induce the neurotoxic A1 phenotype in astrocytes [40] vs. activating the integrin-N-cadherin pathway using type 1 collagen to induce scar-forming astrocytes depending on the focus of the assay [26]. And finally, the quantification of the expression levels of GFAP and proteoglycans have been the most common parameters evaluated to assess the increase or decrease in the reactivity of astrocytes. However, considering the complexity of the process of reactive astrogliosis, an in-depth analysis should be conducted to assess the state of astrocytes rather than relying on a couple of markers. As our understanding of astrocyte biology evolves, assessment of the astrocyte response should include an array of cytoskeletal proteins, growth factors, cytokines, extracellular matrix proteins, ion transporters, and neurotransmitter channels using techniques to study changes at both cellular (ex. flow cytometry) and molecular level (ex. RNA sequencing).
Three-dimensional printing of astrocytes
Three-dimensional printing offers unique control over cellular placement and the architectural shape of the in vitro environment that standard culture practices do not afford [70]. Some unique system controls that three-dimensional printing allows include: 1) exact intercellular distances in cultures containing multiple cell types, 2) precise placement of mechanically distinct materials, extracellular matrix molecules, and/or soluble factors, and 3) three-dimensional scaffold architectures with current resolutions near 50 μm. Using these fabrication techniques, researchers have begun to develop in vitro models to study glioblastomas and connectivity of the nervous system [71,72]. Moving forward, culture systems that model phenotypically relevant astrocytes for the study of the tripartite synapse, astrocyte nutrient uptake from blood vessels, and reactive astrocytes found in disease or after trauma can be envisioned. As the ability to add more complexities to these systems is established, researchers will begin to create relevant models of astrocyte phenotypes that better mimic their in vivo counterpart’s morphology and functionality.
Summary and future directions
The varying functions of astrocytes in combination with their dynamically changing behavior following injury or during disease progression make it challenging to create relevant in vitro astrocyte culture models. However, due to recent advances in understanding astrocyte biology in addition to the advances made in creating complex biomaterial environments, progress is being made to design substrates that better recapitulate astrocyte morphology and physiology. Future directions will likely include continued development of biomaterials, creating three-dimensional cellular organization that recapitulates the astrocyte density found in vivo, and implementation of co-culture systems that enable understanding of communication between neurons, other glia, and the immune system.
Acknowledgements:
The authors would like to acknowledge the following funding sources: Craig H. Neilsen Foundation Fellowship (468116) and Paralyzed Veterans of America Research Foundation Fellowship (3171) to MKG, NIH R01 (NS092754) and New York State Spinal Cord Injury Review Board Institutional Support Grant (C32245GG) to RJG.
Footnotes
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Filous AR, Silver J: “Targeting astrocytes in CNS injury and disease: A translational research approach.” Prog Neurobiol 2016, 144:173–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morest DK, Silver J: Precursors of neurons, neuroglia, and ependymal cells in the CNS: What are they? Where are they from? How do they get where they are going? Glia 2003, 43:6–18. [DOI] [PubMed] [Google Scholar]
- 3.Malatesta P, Hartfuss E, Götz M: Isolation of radial glial cells by fluorescent-activated cell sorting reveals a neural lineage. Development 2000, 127:5253–5263. [DOI] [PubMed] [Google Scholar]
- 4.Voigt T: Development of glial cells in the cerebral wall of ferrets: Direct tracing of their transformation from radial glia into astrocytes. J Comp Neurol 1989, 289:74–88. [DOI] [PubMed] [Google Scholar]
- 5.Yang HY, Lieska N, Glick R, Shao D, Pappas GD: Expression of 300-kilodalton intermediate filament-associated protein distinguishes human glioma cells from normal astrocytes. Proc Natl Acad Sci U S A 1993, 90:8534–8537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang HY, Lieska N, Shao D, Kriho V, Pappas GD: Immunotyping of radial glia and their glial derivatives during development of the rat spinal cord. J Neurocytol 1993, 22:558–571. [DOI] [PubMed] [Google Scholar]
- 7.Ramón y Cajal S: Histologie du Systeme Nerveux de l’homme et des Vertebres, Vol. 2. 1909. [Google Scholar]
- 8.Miller R, Raff M: Fibrous and protoplasmic astrocytes are distinct classes of glial cells. J Neurosci 1984, 4:585–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bushong EA, Martone ME, Jones YZ, Ellisman MH: Protoplasmic astrocytes in CA1 stratum radiatum occupy separate anatomical domains. J Neurosci 2002, 22:183–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ffrench-Constant C, Miller RH, Kruse J, Schachner M, Raff MC: Molecular specialization of astrocyte processes at nodes of Ranvier in rat optic nerve. J Cell Biol 1986, 102:844–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raine CS: On the association between perinodal astrocytic processes and the node of Ranvier in the C.N.S. J Neurocytol 1984, 13:21–27. [DOI] [PubMed] [Google Scholar]
- 12.Duchesne PY, Gerebtzoff MA, Brotchi J: Four types of reactive astrocytes. Bibl Anat 1981, [PubMed] [Google Scholar]
- 13.Grafe MR, Schoenfeld TA: Radial glial cells in the postnatal olfactory tubercle of hamsters. Dev Brain Res 1982, 4:115–118. [DOI] [PubMed] [Google Scholar]
- 14.Silver J, Lorenz SE, Wahlsten D, Coughlin J: Axonal guidance during development of the great cerebral commissures: Descriptive and experimental studies, in vivo, on the role of preformed glial pathways. J Comp Neurol 1982, 210:10–29. [DOI] [PubMed] [Google Scholar]
- 15.Silver J, Edwards MA, Levitt P: Immunocytochemical demonstration of early appearing astroglial structures that form boundaries and pathways along axon tracts in the fetal brain. J Comp Neurol 1993, 328:415–436. [DOI] [PubMed] [Google Scholar]
- 16.Cregg JM, DePaul MA, Filous AR, Lang BT, Tran A, Silver J: Functional regeneration beyond the glial scar. Exp Neurol 2014, 253:197–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kettenmann H, Verkhratsky A: Neuroglia, der lebende Nervenkitt. Fortschritte der Neurol Psychiatr 2011, 79:588–597. [DOI] [PubMed] [Google Scholar]
- 18.Phillips EC, Croft CL, Kurbatskaya K, O’Neill MJ, Hutton ML, Hanger DP, Garwood CJ, Noble W: Astrocytes and neuroinflammation in Alzheimer’s disease. Biochem Soc Trans 2014, 42:1321–1325. [DOI] [PubMed] [Google Scholar]
- 19.Volterra A, Meldolesi J: Astrocytes, from brain glue to communication elements: The revolution continues. Nat Rev Neurosci 2005, 6:626–640. [DOI] [PubMed] [Google Scholar]
- 20.Syková E, Chvátal A: Extracellular ionic and volume changes: The role in glia-Neuron interaction. J Chem Neuroanat 1993, 6:247–260. [DOI] [PubMed] [Google Scholar]
- 21.Müller HW, Matthiessen HP, Schmalenbach C, Schroeder WO: Glial support of CNS neuronal survival, neurite growth and regeneration. Restor Neurol Neurosci 1991, 2:229–232. [DOI] [PubMed] [Google Scholar]
- 22.Yang D, Peng C, Li X, Fan X, Li L, Ming M, Chen S, Le W: Pitx3-transfected astrocytes secrete brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor and protect dopamine neurons in mesencephalon cultures. J Neurosci Res 2008, 86:3393–3400. [DOI] [PubMed] [Google Scholar]
- 23.Gengatharan A, Bammann RR, Saghatelyan A: The role of astrocytes in the generation, migration, and integration of new neurons in the adult olfactory bulb. Front Neurosci 2016, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Theodosis DT, Piet R, Poulain DA, Oliet SHR: Neuronal, glial and synaptic remodeling in the adult hypothalamus: Functional consequences and role of cell surface and extracellular matrix adhesion molecules. Neurochem Int 2004, 45:491–501. [DOI] [PubMed] [Google Scholar]
- 25.Anderson MA, Ao Y, Sofroniew M V.: Heterogeneity of reactive astrocytes. Neurosci Lett 2014, 565:23–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hara M, Kobayakawa K, Ohkawa Y, Kumamaru H, Yokota K, Saito T, Kijima K, Yoshizaki S, Harimaya K, Nakashima Y, et al. : Interaction of reactive astrocytes with type i collagen induces astrocytic scar formation through the integrin-N-cadherin pathway after spinal cord injury. Nat Med 2017, 23:818–828. [DOI] [PubMed] [Google Scholar]
- 27.Wanner IB, Anderson MA, Song B, Levine J, Fernandez A, Gray-Thompson Z, Ao Y, Sofroniew M V.: Glial Scar Borders Are Formed by Newly Proliferated, Elongated Astrocytes That Interact to Corral Inflammatory and Fibrotic Cells via STAT3-Dependent Mechanisms after Spinal Cord Injury. J Neurosci 2013, 33:12870–12886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Faulkner JR: Reactive Astrocytes Protect Tissue and Preserve Function after Spinal Cord Injury. J Neurosci 2004, 24:2143–2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eng LF: Glial fibrillary acidic protein (GFAP): the major protein of glial intermediate filaments in differentiated astrocytes. J Neuroimmunol 1985, 8:203–214. [DOI] [PubMed] [Google Scholar]
- 30.Barrett CP, Guth L, Donati EJ, Krikorian JG: Astroglial reaction in the gray matter of lumbar segments after midthoracic transection of the adult rat spinal cord. Exp Neurol 1981, 73:365–377. [DOI] [PubMed] [Google Scholar]
- 31.Rothermundt M, Peters M, Prehn JHM, Arolt V: S100B in brain damage and neurodegeneration. Microsc Res Tech 2003, 60:614–632. [DOI] [PubMed] [Google Scholar]
- 32.Miyake T, Hattori T, Fukuda M, Kitamura T, Fujita S: Quantitative studies on proliferative changes of reactive astrocytes in mouse cerebral cortex. Brain Res 1988, 451:133–138. [DOI] [PubMed] [Google Scholar]
- 33.Clarke SR, Shetty AK, Bradley JL, Turner DA: Reactive astrocytes express the embryonic intermediate neurofilament nestin. Neuroreport 1994, 5:1885–1888. [DOI] [PubMed] [Google Scholar]
- 34.Rathore KI, Berard JL, Redensek A, Chierzi S, Lopez-Vales R, Santos M, Akira S, David S: Lipocalin 2 Plays an Immunomodulatory Role and Has Detrimental Effects after Spinal Cord Injury. J Neurosci 2011, 31:13412–13419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bi F, Huang C, Tong J, Qiu G, Huang B, Wu Q, Li F, Xu Z, Bowser R, Xia X-G, et al. : Reactive astrocytes secrete lcn2 to promote neuron death. Proc Natl Acad Sci 2013, 110:4069–4074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dyck SM, Karimi-Abdolrezaee S: Chondroitin sulfate proteoglycans: Key modulators in the developing and pathologic central nervous system. Exp Neurol 2015, 269:169–187. [DOI] [PubMed] [Google Scholar]
- 37.Yiu G, He Z: Glial inhibition of CNS axon regeneration. Nat Rev Neurosci 2006, 7:617–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jin X, Yamashita T: Microglia in central nervous system repair after injury. J Biochem 2016, 159:491–496. [DOI] [PubMed] [Google Scholar]
- 39.Zamanian JL, Xu L, Foo LC, Nouri N, Zhou L, Giffard RG, Barres BA: Genomic Analysis of Reactive Astrogliosis. J Neurosci 2012, 32:6391–6410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L, Bennett ML, Münch AE, Chung W-S, Peterson TC, et al. : Neurotoxic reactive astrocytes are induced by activated microglia. Nature 2017, 541:481–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li K, Li J, Zheng J, Qin S: Reactive Astrocytes in Neurodegenerative Diseases. Aging Dis 2019, 10:664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lange SC, Bak LK, Waagepetersen HS, Schousboe A, Norenberg MD: Primary cultures of astrocytes: Their value in understanding astrocytes in health and disease. Neurochem Res 2012, 37:2569–2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sofroniew M V, Vinters H V: Astrocytes: Biology and pathology. Acta Neuropathol 2010, 119:7–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Codeluppi S, Gregory EN, Kjell J, Wigerblad G, Olson L, Svensson CI: Influence of rat substrain and growth conditions on the characteristics of primary cultures of adult rat spinal cord astrocytes. J Neurosci Methods 2011, 197:118–127. [DOI] [PubMed] [Google Scholar]
- 45.Saura J: Microglial cells in astroglial cultures: A cautionary note. J Neuroinflammation 2007, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.TCW J, Wang M, Pimenova AA, Bowles KR, Hartley BJ, Lacin E, Machlovi SI, Abdelaal R, Karch CM, Phatnani H, et al. : An Efficient Platform for Astrocyte Differentiation from Human Induced Pluripotent Stem Cells. Stem Cell Reports 2017, 9:600–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Swartzlander DB, Propson NE, Roy ER, Saito T, Saido T, Wang B, Zheng H: Concurrent cell type-specific isolation and profiling of mouse brains in inflammation and Alzheimer’s disease. JCI insight 2018, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang Y, Sloan SA, Clarke LE, Caneda C, Plaza CA, Blumenthal PD, Vogel H, Steinberg GK, Edwards MSB, Li G, et al. : Purification and Characterization of Progenitor and Mature Human Astrocytes Reveals Transcriptional and Functional Differences with Mouse. Neuron 2016, 89:37–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ben Haim L, Rowitch DH: Functional diversity of astrocytes in neural circuit regulation. Nat Rev Neurosci 2016, doi: 10.1038/nrn.2016.159. [DOI] [PubMed] [Google Scholar]
- 50.Curtis A, Wilkinson C: Topographical control of cells. Biomaterials 1997, 18:1573–1583. [DOI] [PubMed] [Google Scholar]
- 51.Cao H, Marcy G, Goh ELK, Wang F, Wang J, Chew SY: The Effects of Nanofiber Topography on Astrocyte Behavior and Gene Silencing Efficiency . Macromol Biosci 2012, doi: 10.1002/mabi.201100436. [DOI] [PubMed] [Google Scholar]
- 52.Puschmann TB, Zandén C, De Pablo Y, Kirchhoff F, Pekna M, Liu J, Pekny M: Bioactive 3D cell culture system minimizes cellular stress and maintains the in vivo-like morphological complexity of astroglial cells. Glia 2013, 61:432–440. [DOI] [PubMed] [Google Scholar]
- 53.Mattotti M, Alvarez Z, Ortega JA, Planell JA, Engel E, Alcántara S: Inducing functional radial glia-like progenitors from cortical astrocyte cultures using micropatterned PMMA. Biomaterials 2012, 33:1759–1770. [DOI] [PubMed] [Google Scholar]
- 54.Zuidema JM, Desmond GP, Rivet CJ, Kearns KR, Thompson DM, Gilbert RJ: Nebulized solvent ablation of aligned PLLA fibers for the study of neurite response to anisotropic-to-isotropic fiber/film transition (AFFT) boundaries in astrocyte-neuron co-cultures. Biomaterials 2015, 46:82–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Johnson CDLL, Zuidema JM, Kearns KR, Maguire AB, Desmond GP, Thompson DM, Gilbert RJ: The Effect of Electrospun Fiber Diameter on Astrocyte-Mediated Neurite Guidance and Protection. ACS Appl Bio Mater 2019, 2:104–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lau CL, Kovacevic M, Tingleff TS, Forsythe JS, Cate HS, Merlo D, Cederfur C, Maclean FL, Parish CL, Horne MK, et al. : 3D Electrospun scaffolds promote a cytotrophic phenotype of cultured primary astrocytes. J Neurochem 2014, 130:215–226. [DOI] [PubMed] [Google Scholar]
- 57.Min SK, Kim SH, Kim CR, Paik SM, Jung SM, Shin HS: Effect of topography of an electrospun nanofiber on modulation of activity of primary rat astrocytes. Neurosci Lett 2013, 534:80–84. [DOI] [PubMed] [Google Scholar]
- 58.Gottipati MK, Samuelson JJ, Kalinina I, Bekyarova E, Haddon RC, Parpura V: Chemically functionalized single-walled carbon nanotube films modulate the morpho-functional and proliferative characteristics of astrocytes. Nano Lett 2013, 13:4387–4392. [DOI] [PubMed] [Google Scholar]
- 59.Zuidema JM, Hyzinski-García MC, Van Vlasselaer K, Zaccor NW, Plopper GE, Mongin AA, Gilbert RJ: Enhanced GLT-1 mediated glutamate uptake and migration of primary astrocytes directed by fibronectin-coated electrospun poly-l-lactic acid fibers. Biomaterials 2014, 35:1439–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hwang B, Lee JH, Bang D: Single-cell RNA sequencing technologies and bioinformatics pipelines. Exp Mol Med 2018, doi: 10.1038/s12276-018-0071-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Grant R, Hallett J, Forbes S, Hay D, Callanan A: Blended electrospinning with human liver extracellular matrix for engineering new hepatic microenvironments. Sci Rep 2019, doi: 10.1038/s41598-019-42627-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zuidema JM, Kumeria T, Kim D, Kang J, Wang J, Hollett G, Zhang X, Roberts DS, Chan N, Dowling C, et al. : Oriented Nanofibrous Polymer Scaffolds Containing Protein-Loaded Porous Silicon Generated by Spray Nebulization. Adv Mater 2018, 30:1706785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Molina ER, Chim LK, Salazar MC, Mehta SM, Menegaz BA, Lamhamedi-Cherradi SE, Satish T, Mohiuddin S, McCall D, Zaske AM, et al. : Mechanically tunable coaxial electrospun models of YAP/TAZ mechanoresponse and IGF-1R activation in osteosarcoma. Acta Biomater 2019, doi: 10.1016/j.actbio.2019.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kozai TDY, Jaquins-Gerstl AS, Vazquez AL, Michael AC, Cui XT: Brain tissue responses to neural implants impact signal sensitivity and intervention strategies. ACS Chem Neurosci 2015, 6:48–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Taylor SJ, Rosenzweig ES, McDonald JW, Sakiyama-Elbert SE: Delivery of neurotrophin-3 from fibrin enhances neuronal fiber sprouting after spinal cord injury. J Control Release 2006, 113:226–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Khaing ZZ, Milman BD, Vanscoy JE, Seidlits SK, Grill RJ, Schmidt CE: High molecular weight hyaluronic acid limits astrocyte activation and scar formation after spinal cord injury. J Neural Eng 2011, 8. [DOI] [PubMed] [Google Scholar]
- 67.Anderson MA, O’Shea TM, Burda JE, Ao Y, Barlatey SL, Bernstein AM, Kim JH, James ND, Rogers A, Kato B, et al. : Required growth facilitators propel axon regeneration across complete spinal cord injury. Nature 2018, 561:396–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Galarza S, Crosby AJ, Pak CH, Peyton SR: Control of Astrocyte Quiescence and Activation in a Synthetic Brain Hydrogel. Adv Healthc Mater 2020, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bowers HC, Fiori ML, Khadela JB, Janmey PA, Galie PA: Cell-matrix tension contributes to hypoxia in astrocyte-seeded viscoelastic hydrogels composed of collagen and hyaluronan. Exp Cell Res 2019, 376:49–57. [DOI] [PubMed] [Google Scholar]
- 70.Fantini V, Bordoni M, Scocozza F, Conti M, Scarian E, Carelli S, Di Giulio AM, Marconi S, Pansarasa O, Auricchio F, et al. : Bioink Composition and Printing Parameters for 3D Modeling Neural Tissue. Cells 2019, 8:830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Heinrich MA, Bansal R, Lammers T, Zhang YS, Michel Schiffelers R, Prakash J: 3D-Bioprinted Mini-Brain: A Glioblastoma Model to Study Cellular Interactions and Therapeutics. Adv Mater 2019, doi: 10.1002/adma.201806590. [DOI] [PubMed] [Google Scholar]
- 72.Johnson BN, Lancaster KZ, Hogue IB, Meng F, Kong YL, Enquist LW, McAlpine MC: 3D printed nervous system on a chip. Lab Chip 2016, doi: 10.1039/c5lc01270h. [DOI] [PMC free article] [PubMed] [Google Scholar]