Figure 6.
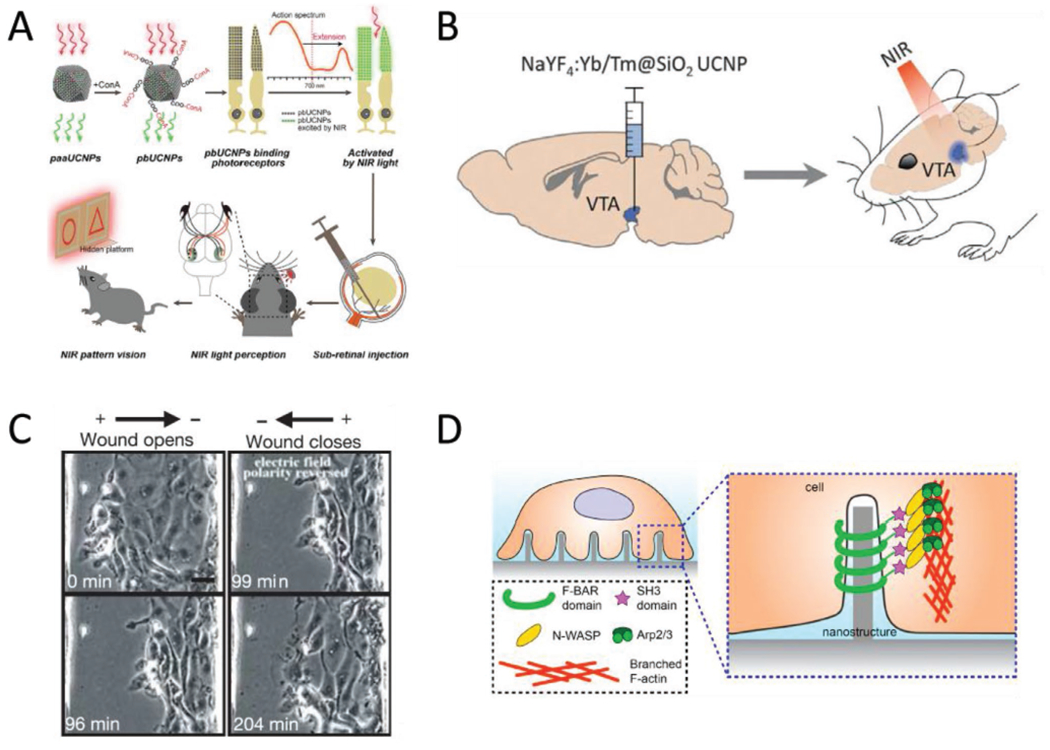
Other biointerface devices for enacting systemic changes. A) Schematic of the UCNP-based method for enabling NIR vision in rats. The UCNPs absorb at 950 nm and emit at 535 nm. Injections of surface modified UCNPs to the sub-retinal space eventually produces “nanoantannae,” hybrids of the UCNPs and rod optical sensors. Subsequent training and experiments indicated the injected rats could discern NIR light, while control groups could not. Reproduced with permission.[46] 2019, Cell Press. B) Optogenetic techniques can be expanded to deep tissue stimulation by pairing optogenetic constructs with UCNPs. The ability of UCNPs to convert NIR light to the visible region resolves the incompatibility with the light scattering effect of tissues and the existing range of wavelengths that can actuate optogenetic proteins. Adding UCNPs to the protocol means that NIR light, which has the optimal tissue penetration, can be converted to blue light, which activates channelrhodopsin, deep in the brain. Adapted with permission.[78] Copyright 2018, AAAS. C) Electric fields at physiological magnitudes (150 mV mm−1) impact the progress of wound healing in epithelial cells—an example of bioelectronics impacting non-excitable cell types. In this instance, the polarity of the externally applied field determined whether the opening would open or close over time. Scale bar = 20 μm. Reproduced with permission.[75] Copyright 2006, Springer Nature. D) Geometry as a possible avenue for device-based targeted modulation. Nanoscale surface features can trigger the cytoskeletal protein actin to accumulate at regions of curvature when the radius is under the 400 nm threshold. The curvature-dependent actin accumulation is indicative of the possibility for devices that take advantage of both electrical and morphological cues, provided respectively by the device functionality and the geometry of the implant. Reproduced with permission.[119] Copyright 2019, National Academy of Sciences.
