Figure 2. Soft–Hard Composites Can Be Categorized for Bioelectric Interfaces.
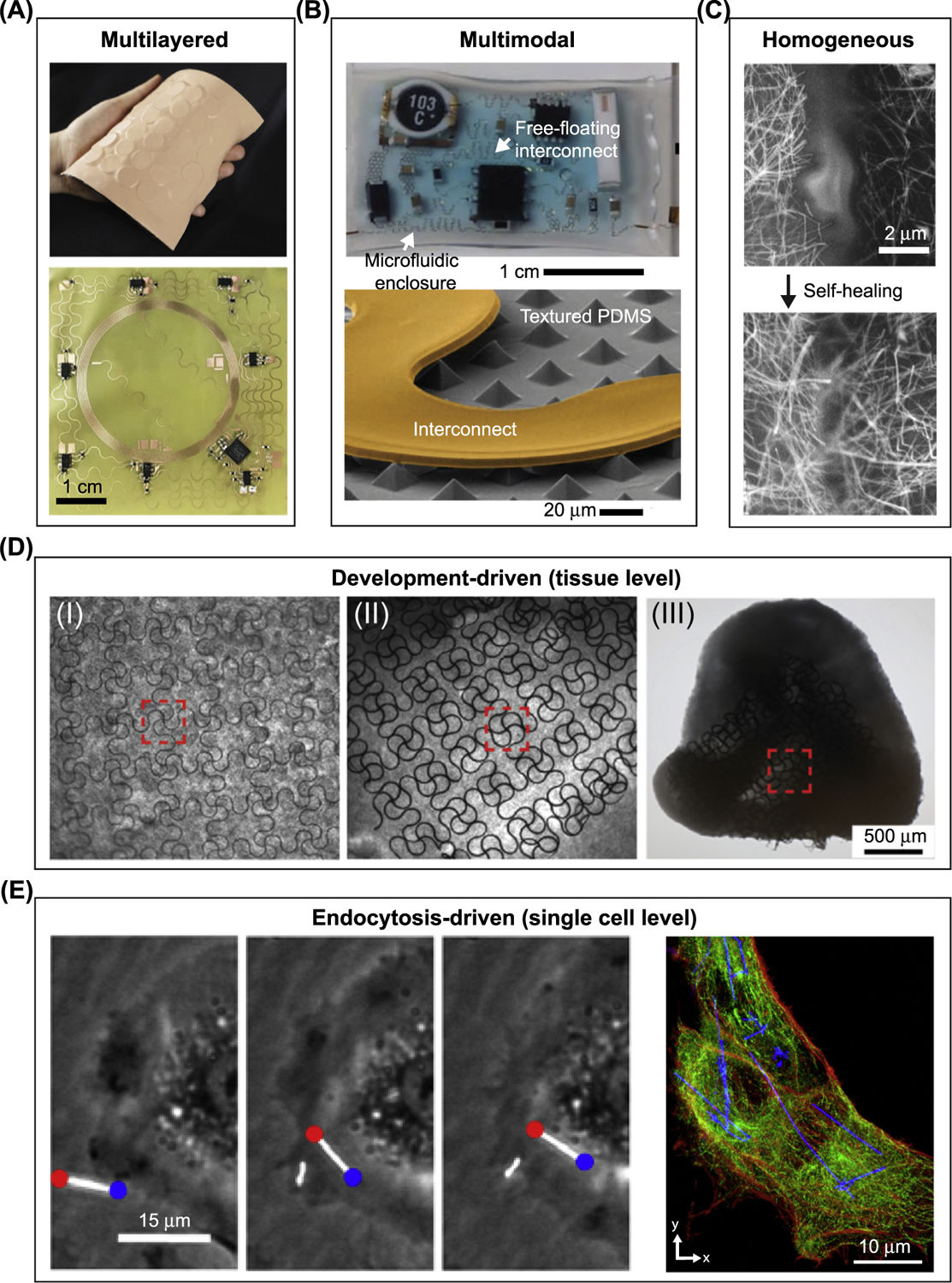
(A–C) Soft–hard composites enabled by synthetic materials. (A) Multilayered interface: (top) photograph of an epidermal virtual reality device, which integrates silicon, actuators, electronics, a Cu-based near-field communication coil (NFC), and polyimide. (Bottom) Optical image shows the NFC coil after integrating the electronic components. Adapted, with permission, from [5]. (B) Multimodal interface: (top) photograph of a soft microfluidic assembly of sensors, circuits, and radios used as epidermal electronics. (Bottom) Scanning electron microscopy (SEM) image of part of the internal device shows a textured polydimethylsiloxane (PDMS) surface and the free-standing interconnect. Liquid can be injected to help impact absorption. Adapted, with permission, from [6]. (C) Homogeneous interface: SEM images of a broken Ag nanowire/PDMS–MPU0.4–IU0.6 composite conductor before (top) and after (bottom) self-healing. Adapted, with permission, from [9]. (D,E) Biology-driven formation of the soft–hard composites. (D) Mechanobiology-driven interface: optical microscopy images of different stages in ‘cyborg’ cardiac organoid formation show the organogenesis force-induced deformation and 3D incorporation of mesh-like nanoelectronics. Adapted, with permission, from [11]. (E) Endocytosis-driven interface: (left) scatter-enhanced phase-contrast images show the internalization of Si nanowires into the human umbilical vein endothelial cell (HUVEC). (Right) confocal fluorescence microscopy images of a silicon nanowire/HUVEC composite shows intracellular distribution of Si nanowires. Actin, red; tubulin, green. Adapted, with permission, from [14]. Abbreviations: IU, Isophorone bisurea unit; MPU, 4,4′ -methylenebis(phenyl urea) unit.
