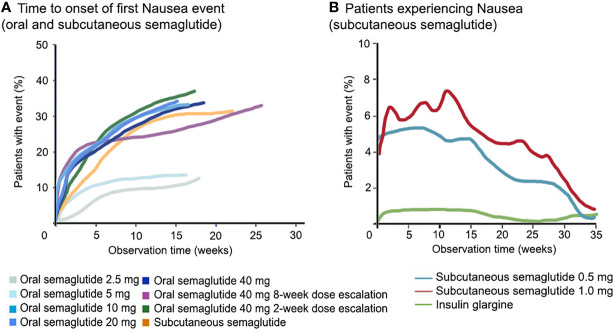
Figure 2.
Course of nausea with semaglutide. GLP-1RA, including semaglutide, cause nausea in about one third of treated patients, which is both dose- and time-dependent. In panel (A), a direct comparison between subcutaneous and oral semaglutide is shown, as well as different doses of oral semaglutide, for the first occurrence of nausea. In panel (B), the course of the occurrence of nausea is shown for subcutaneous semaglutide. Data for panel (A) are derived from the phase-2 trial (38), for panel (B) data are shown from (26). GLP-1RA, glucagon-like peptide-1 receptor agonist.