Figure 2.
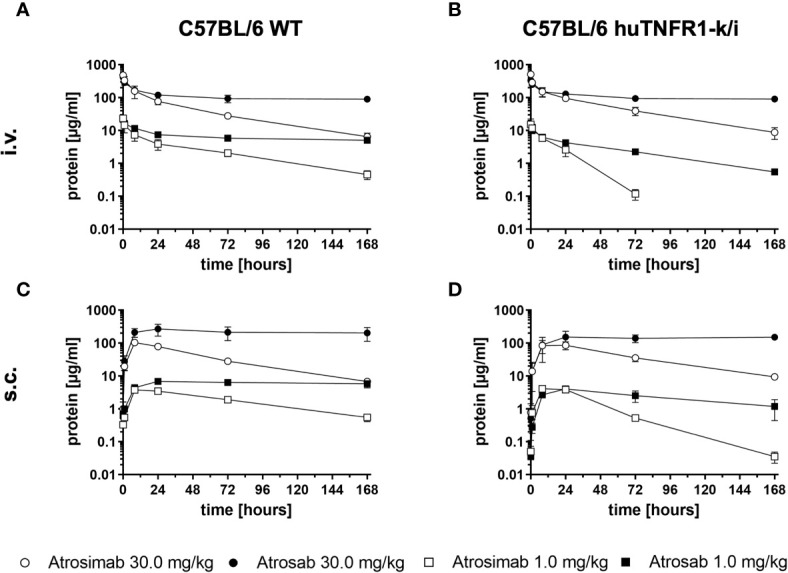
Comparative pharmacokinetic analysis of Atrosimab and ATROSAB. Atrosimab or Atrosab were injected (A, B) intravenously (i.v.) or (C, D) subcutaneously (s.c.) at doses of 1.0 mg/kg or 30.0 mg/kg body weight into (A, C) C57BL/6 wildtype (WT) or (B, D) C57BL/6 huTNFR1-k/i mice. Serum samples, collected at the indicated time points, were analyzed for remaining circulating protein by ELISA. Shown are the mean ± SD of three mice.
