Figure 1.
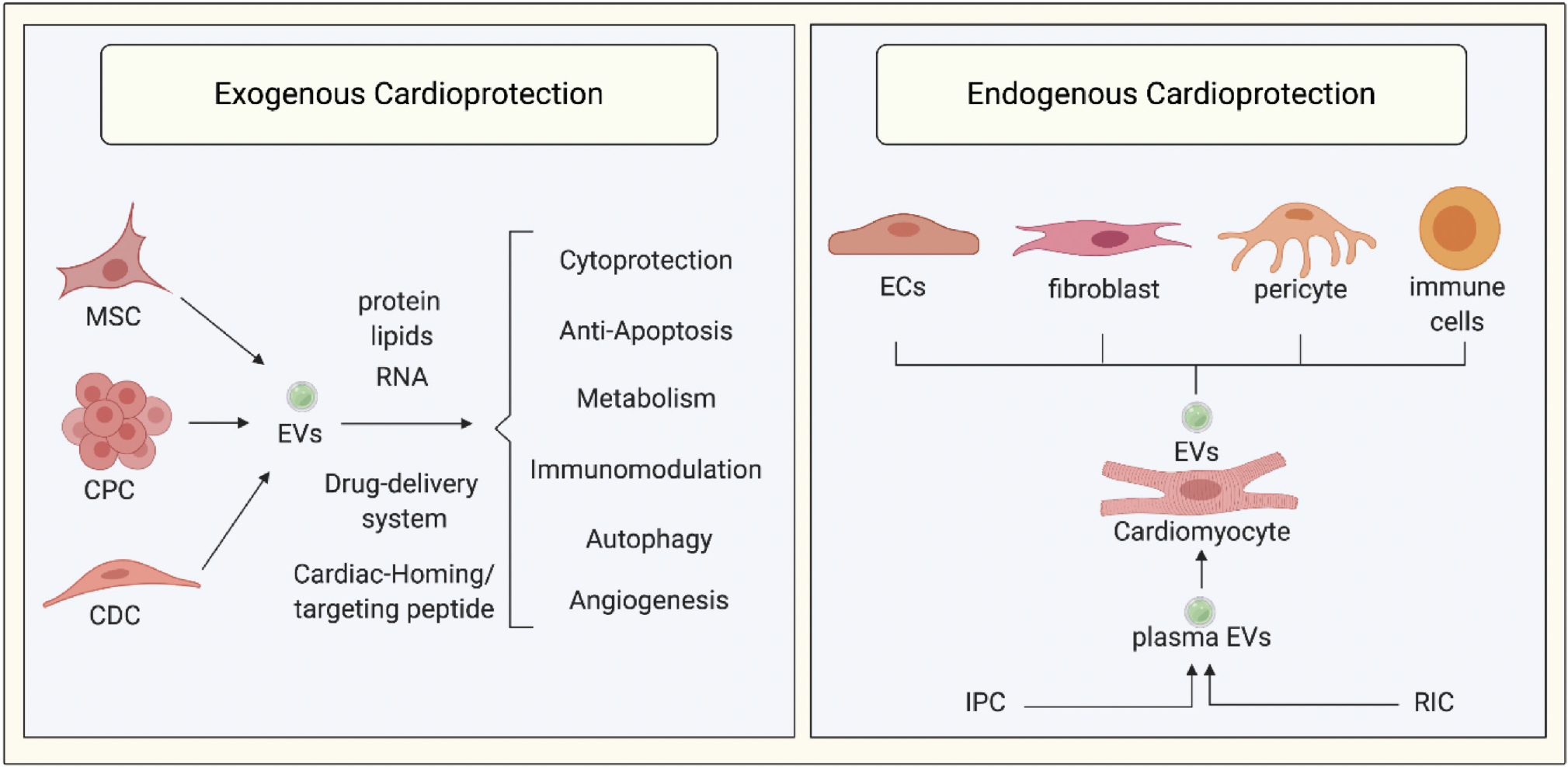
Extracellular vesicles and cardioprotection. Extracellular vesicles (EVs) are known to act a mediators of endogenous cardioprotection by mediating intercellular communication between cardiomyocytes and endothelial cells (ECs), fibroblasts, pericytes, and immune cells. In addition, plasma EVs have been shown to mediate the cardioprotection elicited by endogenous strategies such as ischemic preconditioning (IPC) or remote ischemic conditioning (RIC). Stem cell-derived EVs from mesenchymal stromal cells (MSC), cardiac progenitor cells (CPC), and cardiosphere derived cells (CDC) have been shown to reduce myocardial infarct size and to prevent adverse left ventricular remodeling in small and large animal infarct models through a variety of mechanisms including cytoprotection, anti-apoptotic effects, metabolic effects, immunomodulation, increasing autophagy, and enhancing angiogenesis. Stem cell-derived EVs can be used as drug delivery systems to deliver cardioprotective drugs to the ischemic heart following acute myocardial infarction. Finally, addition of cardiac homing/targeting peptides to stem cell-derived EVs can target them to the ischemic heart, thereby increasing bioavailability and cardioprotective efficacy.
