Figure 4.
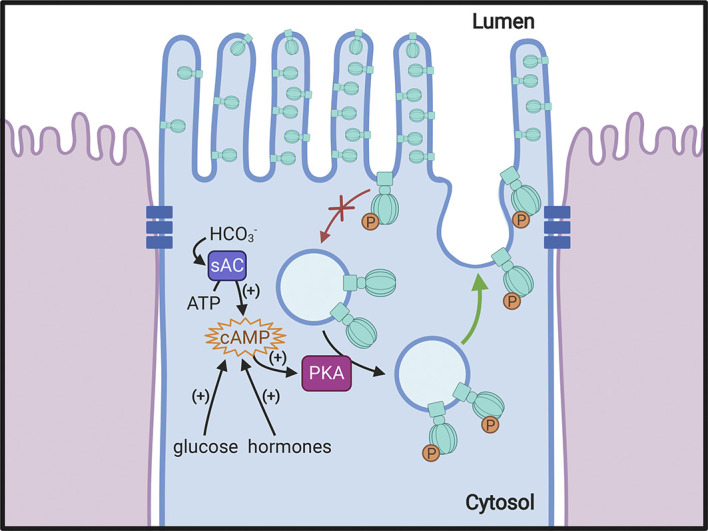
Regulation of V-ATPase by vesicle recycling. Recycling of the V-ATPase between intracellular vesicles and the plasma membrane is a mechanism by which proton secretion is modulated. Incorporation of V-ATPase into the apical membrane occurs in response to various physiological stimuli, including several hormones and glucose. One common feature of the process in different cell types is the activation of PKA by increased intracellular cAMP, resulting in phosphorylation (P) of some V-ATPase subunits, including A and C in the cytosolic V1 sector. By an unknown mechanism, this leads to an alteration of the balance between exocytosis (stimulated: green arrow) and endocytosis (reduced: red arrow) of V-ATPase-rich vesicles, resulting in a net accumulation of plasma membrane V-ATPase. One potential stimulus in the kidney and in the epididymis is bicarbonate, generated either by direct entry into the cell through apical bicarbonate exchangers or by the activity of carbonic anhydrase type II in the cytosol. The increased concentration stimulates the production of cAMP by soluble adenylyl cyclase (sAC), thereby activating PKA. Phosphorylated V-ATPase complexes are shown at a larger size for clarity. Created with BioRender.com.
