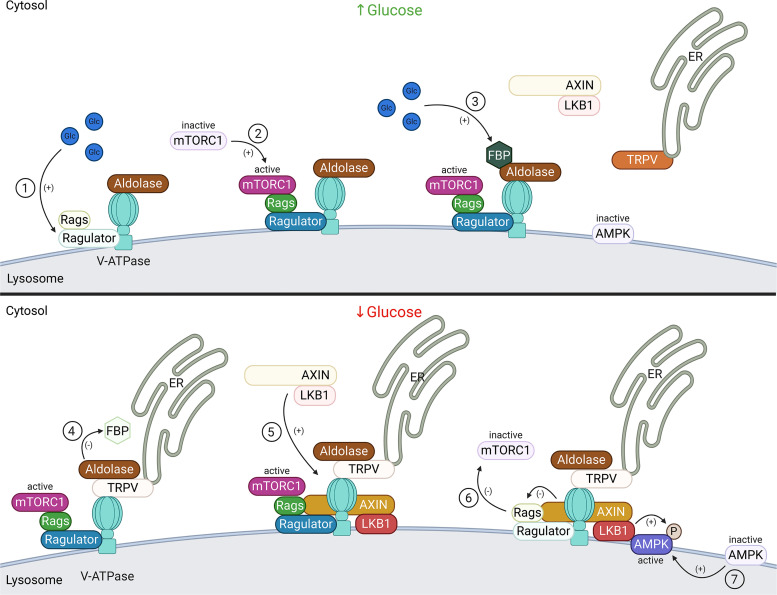
Figure 10.
V-ATPase-dependent glucose signaling. When glucose is present (top), it activates the Ragulator complex, which acts as a GEF toward the Rag GTPases (Rags), activating them (1). The Rag GTPases are anchored to the lysosomal membrane by the Ragulator complex bound to the V-ATPase. Subsequently, mammalian target of rapamycin complex 1 (mTORC1) is recruited to the lysosomal membrane by the active Rag GTPases (2), where it can be activated by the lysosomal Rheb GTPases (see Fig. 9). Additionally, when glucose is present, the glycolytic intermediate fructose-1,6-bisphosphate (FBP) binds to the aldolase-V-ATPase complex (3). Under conditions of glucose starvation (bottom), FBP disassociates from aldolase allowing endoplasmic reticulum (ER)-localized transient receptor potential V-type (TRPV) channels to bind the V-ATPase at the aldolase binding site (4). FBP-unoccupied aldolase binds and inhibits TRPV Ca2+ channel activity allowing for recruitment of the AXIN-LKB1 complex to the lysosomal membrane (5) where it interacts with Ragulator and inhibits its GEF activity toward Rags causing the disassociation from the lysosome and inactivation of mTORC1 (6). Furthermore, the V-ATPase, AXIN, and LKB1 recruit AMPK to form a super complex where AMPK is phosphorylated and activated by LKB1 (7). Created with BioRender.com.