Figure 3.
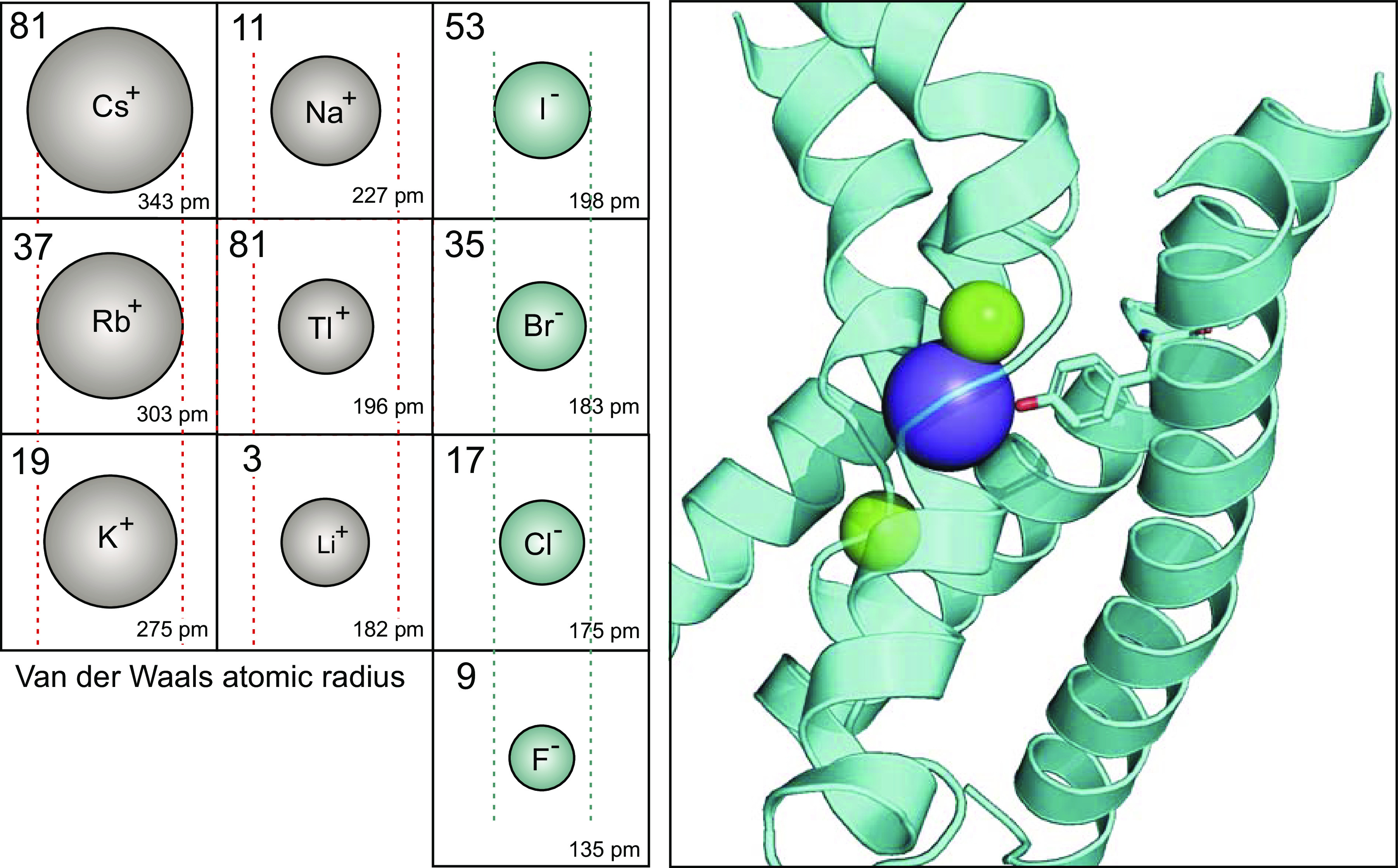
Atomic sizes of monovalent cations and anions. Left: for each ion, the atomic number is located in the top left corner, while the atomic radius (Van der Waals) in picometer (pm, or 10−12 m) is placed in the bottom right corner. The size of the drawn ion is proportional to its atomic radius. Lines (red) are placed around the size of the Rb+ ion, which is transported by Na-K-2Cl and K-Cl cotransporters. Cs+ is much larger, whereas K+ has an equivalent size. Na+, Tl+, and Li+ are much smaller. Note that cations are alkali metals (column 1 in periodic table), except for Thallium, which is a post-transition metal (column 13 in periodic table). The anions are generally smaller with I− of a size similar to Na+. Br− and Cl− are slightly smaller and Fl− is much smaller than I−. The atomic radius values were obtained from the Los Alamos National Laboratories (https://periodic.lanl.gov/list.shtml). Right: ribbon structure of portions of TM1, TM3, and TM6 showing the location of ions in the cryo-EM structure of hKCC1, PDB ID: 6KKT (37). K+ is in blue, whereas Cl− ions are in green. The tyrosine residue located in TM3 which coordinate K+ binding is drawn as stick.
