Figure 1.
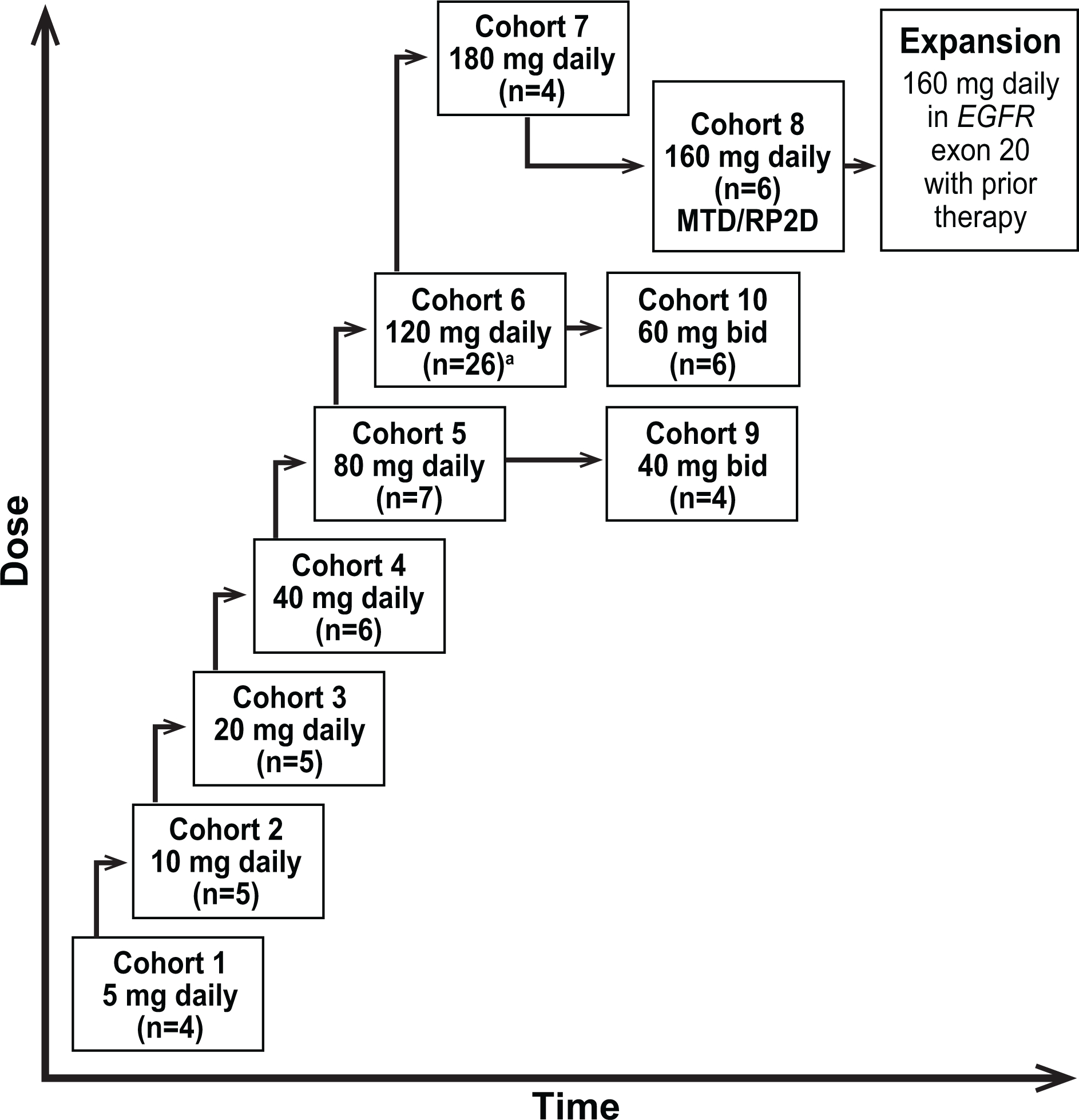
Schema for the dose-escalation phase of the phase 1/2 trial of mobocertinib. The dose-escalation phase followed a conventional 3+3 design. The dose level for each new cohort was up to 100% higher than the dose level in the previous cohort until a grade 2 drug-related toxicity of diarrhea or skin rash occurred, based on expected class effects for EGFR TKIs, or until other DLTs were identified. Further dose escalation involved increments of ≤50% of the previous dose, depending on safety findings
Abbreviations: bid, twice daily; DLTs, dose-limiting toxicities; EGFR, epidermal growth factor receptor; EGFR, epidermal growth factor receptor gene; MTD, maximum tolerated dose; daily, once daily; RP2D, recommended phase 2 dose
a Seven patients were enrolled in the dose escalation to evaluate DLT; additional patients were included to further confirm safety observations.
