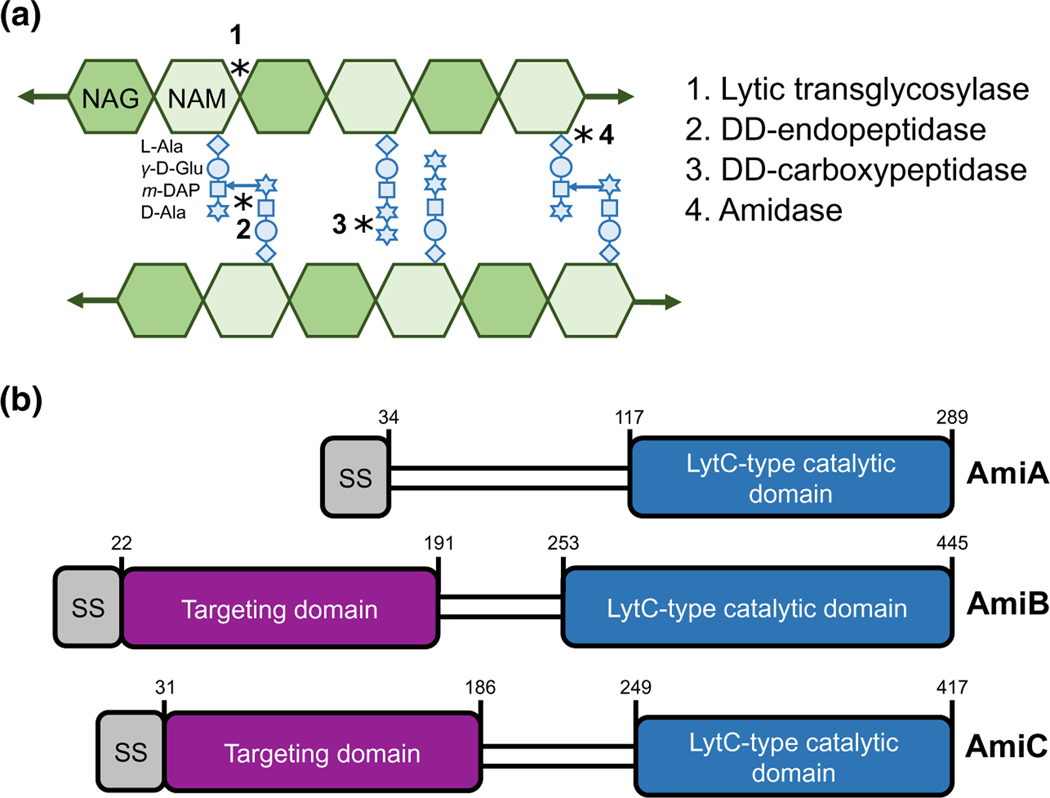
FIGURE 1.
E. coli produces three LytC-type-N-acetylmuramoyl-L-alanine amidases. (a) A model of the peptidoglycan sacculus. N-acetylglucosamine (GlcNAc) and N-acetylmuramic acid (MurNAc) sugars conjoined by a β-(1,4) linkage form glycan strands. Peptide stems emanate from the MurNAc and may be crosslinked to adjacent glycan strands. Peptidoglycan hydrolases cleave the bonds indicated by asterisks. (b) E. coli produces three LytC-type-N-acetylmuramoyl-L-alanine amidases involved in cell separation. Schematic depicts signal sequence (grey), septum targeting domain (purple), and LytC-type catalytic domain (blue). Note that AmiA and AmiC are targeted through the periplasm through the twin arginine translocation (Tat) protein export pathway, whereas AmiB is targeted through the Sec protein translocation pathway