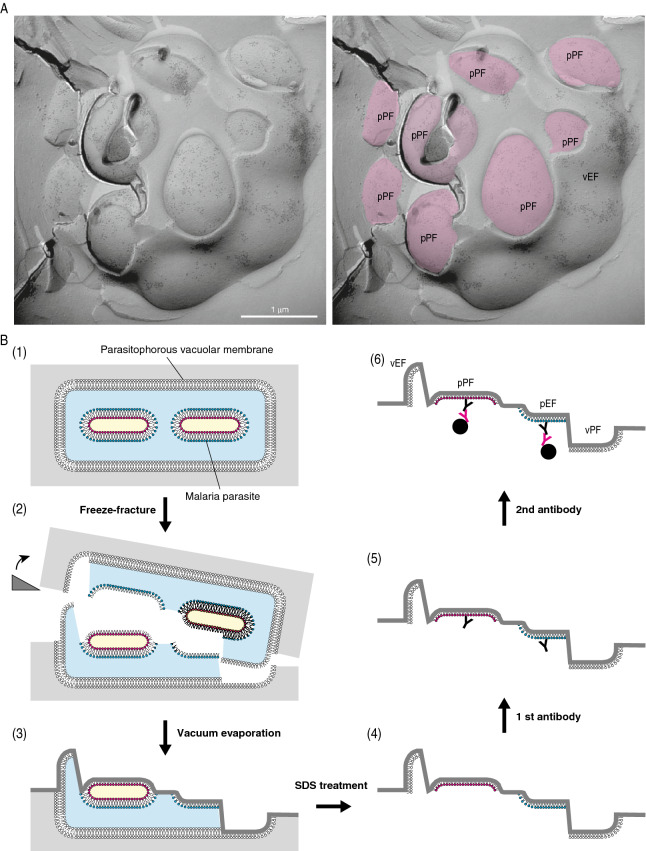
Figure 2.
Outline of the QF-FRL method and GM3 localization in the membrane of live P. falciparum. (A) Freeze-fracture planes of P. falciparum at the schizonts stage consisting of maturing merozoites enclosed in a PV, comprising a single membrane bilayer. The P-faces are adjacent to the cytoplasm, and the E-faces to the exterior side. The labeling of anti-GM3 antibody was localized on both the P. falciparum EF (pEF) and P. falciparum PFs (pPF). In panel in A, pink represents the PF of the plasma membrane in P. falciparum. Scale bar: 1 μm. (B) Outline of the QF-FRL method in the P. falciparum-infected human erythrocyte. (1) QF: Live cells were quickly frozen without ice crystal formation. The metal contact freezing method was used in the present study. (2) Freeze-fracture: Frozen P. falciparum cells in human erythrocytes were fractured at below − 130 °C and under a high vacuum. Membranes were split into two leaflets, and the hydrophobic interface (i.e., the acyl chain side of the phospholipid monolayer) was exposed. (3) Vacuum evaporation: thin layers of carbon and platinum were deposited onto the hydrophobic interface of membranes to physically stabilize the molecules. Because platinum was evaporated at an oblique angle to the specimen’s surface (45°), protruding structures block the evaporating atoms to produce “shadows” behind the structures. Areas deficient in the platinum deposition, therefore, appeared to be electron-lucent under EM. Transmembrane proteins were seen as small bumps termed IMPs. (4) SDS treatment: Specimens were thawed and treated with an SDS solution to dissolve materials other than the lipid monolayer and integral membrane proteins, which were in direct contact with the carbon and platinum layer. This makes membrane proteins and lipid head groups accessible for antibody labeling (5). To visualize the antibody labeling under an electron microscopy, the first antibody was labeled with colloidal gold-conjugated secondary antibody on the replica specimens (6).