Abstract
Introduction
Dysbaric osteonecrosis, albeit rare, have been reported in patients with decompression sickness. We report a patient with dysbaric osteonecrosis, diagnosed 60 days after presenting with decompression sickness.
Case presentation
A 38-year-old, previously fit and healthy male, noted his tank running out of air at approximately 40–50 m while diving, surfaced rapidly before losing consciousness. Upon gaining consciousness, he noted loss of power on all four limbs. He completed 26 sessions of hyperbaric oxygen treatment. Magnetic resonance (MR) of the spine noted T2 abnormality in the upper cervical spine, with some involvement of the central grey matter and the remainder of the cord. According to the International Standards for Neurological Classification of Spinal Cord Injury, it was noted clinically that the patient had a T9 neurological level with ASIA impairment scale A. MR imaging (MRI) of the shoulder was performed, 60 days since initial presentation, after the patient complained of shoulder pain, noted non-specific subcortical oedema of the humeral head, which suggested early osteonecrosis.
Discussion
Dysbaric osteonecrosis is rare but remains extremely important to be recognised as a potential complication from decompressive sickness. The increased risk of pathological fractures with dysbaric osteonecrosis plays an important role as it may alter the rehabilitation prescription. One of the unique features of this case, apart from its rarity, was that it was diagnosed 60 days from his initial presentation, when he has passed his acute phase of illness.
Subject terms: Disability, Spinal cord diseases
Introduction
Decompression sickness, also known as Caisson’s disease or the ‘Bends’ or decompression illness, is a condition that is noted when there is a quick pressure reduction change, when one is using compressed air, as seen in rapid ascend from a dive. The rapid change in pressure results in the formation of bubbles (mainly nitrogen) which can cause a gas embolism. Occlusion of the vessels and subsequent vessel rupture, or tissue compression, induces endothelial damage and plasma extravasation, activating the clotting and inflammatory cascades [1, 2]. Clinical symptoms may present within minutes of surfacing, and consist of a multitude of different symptoms. Patients may present with fatigue, muscle and joint pain, and in severe cases, brain and spinal cord involvement is seen.
Dysbaric osteonecrosis is a type form avascular necrosis of the bone, which is seen in undersea divers, workers who breath compressed air or gas, or as a late effect of decompression sickness [2].
We report a case of a patient with neurological manifestation secondary to decompression sickness, who later developed dysbaric osteonecrosis, while undergoing rehabilitation at our hyper-acute neurorehabilitation unit (HARU) in a major trauma centre, in the United Kingdom.
Case presentation
A 39-year-old experienced, recreational diver, was out on his 33rd dive, with a group of divers, using compressed air in the waters of Southern Adriatic. When he was in at allegedly 40, possibily 50 m dive, he noted that he ran out of air and rapidly surfaced but lost consciousness during the way up. Fortunately, he was able to signal for help prior to losing consciousness. He remained floating on the surface, and his diving partners alerted the emergency medical services, while providing first aid. He was intubated and directed to the Intensive Care Unit of the local hospital.
He was extubated after 24 h, and 12 h after being extubated, he was able to communicate. There was a global reduction in power in all four limbs, particularly his right arm and leg. He was subsequently transferred to the local recompression unit for hyperbaric oxygen treatment. On arrival to the recompression unit, he was noted to be moderately dyspnoeic with weakness in all four limbs and bradyphrenia.
He underwent 26 sessions of hyperbaric oxygen treatment, using the US Navy Treatment Table 6 (USN TT6). He was subsequently repatriated on a 2-h plane journey to our hospital, which is his local hospital, and was provided with hyper-acute neurorehabilitation.
He was working as a waiter up to his admission, has a smoking history of 5 pack/years, and had no history of excess alcohol intake or recreational drugs. He had no other past medical history of note.
On arrival to our hospital, neurological examination noted a right pronator drift. He had significant problems with spasms, affecting his trunk and lower limb. There were bilateral pressure ulcers on both heels, left worse than the right, with small blisters and serous fluid on the ulcer. There was increased tone on both lower limbs. His examination of his power is as per Table 1. Clinically he had T9 neurological level with ASIA impairment scale A (Table 1). He was isolated in a side room, as per our hospital policy for inter-hospital transfer, due to the COVID infection risk, while waiting for a bed at our hyper-acute neurorehabilitation ward.
Table 1.
International standards for neurological classification of spinal cord injury examination.
His blood results noted a mildly raised white cell count (14.2 × 109/L) and a raised CRP (121.7 mg/L). As he was clinically stable, antibiotics were not commenced, and tissue viability nurse input was obtained.
He was reviewed by our spinal cord injury and spasticity outreach teams, and was noted to have significant spasms, leading to knee flexion at times. His spasms were also painful and affected his sleep. He scored a 3 on the Modified Ashworth Scale. There were several triggers for his significant spasticity (constipation, bladder issue, skin issues), but in view of the severity of it, his anti-spasticity medication was up titrated, and a bowel regime was commenced. He was noted to have started to loose some range of movement at his ankles secondary to his spasticity, however, splinting was not feasible due to his pressure sore, and this was taped by the physiotherapist instead, with regular monitoring to ensure he does not continue to loose his range of movement MR spine at our unit noted well demarcated posterior and lateral cord T2 abnormality in the upper cervical spine, with further widespread, more ill-defined abnormality through the remainder of the cord, involving nearly the entire cord length (Fig. 1). MR brain did not show any abnormality. Neurology opinion was obtained, and the scan finding together with the clinical presentation was felt to be in keeping with myelopathy secondary to decompression sickness.
Fig. 1.
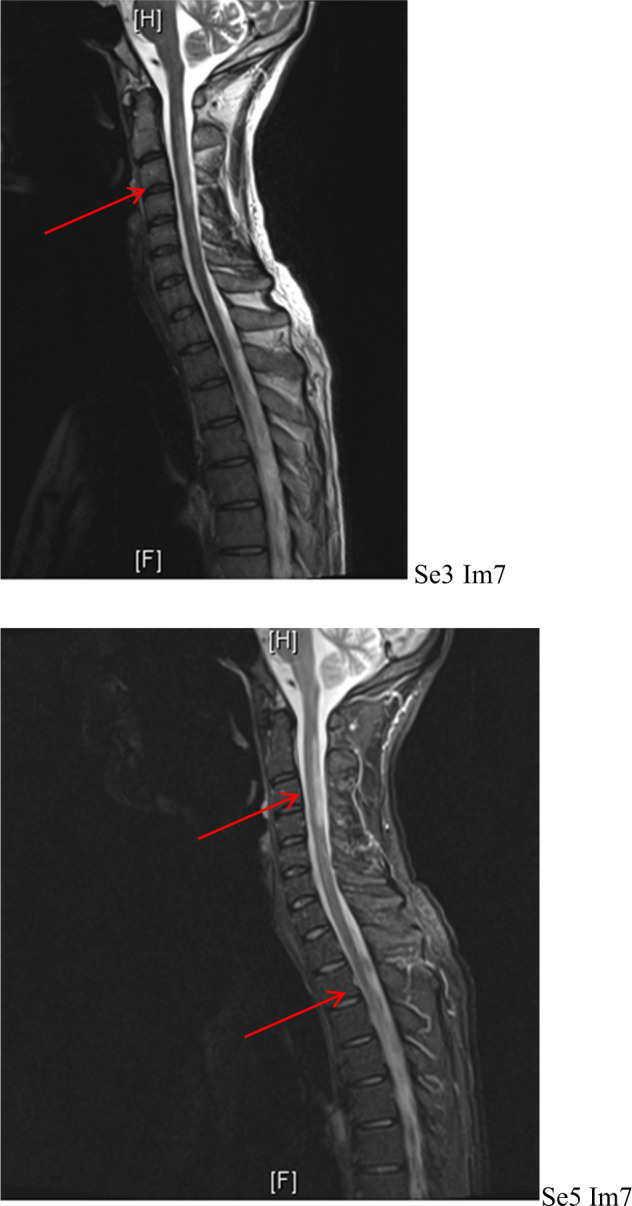
MR spine showing well demarcated posterior and lateral cord T2 abnormality within the upper cervical spine with further widespread, more ill-defined abnormality through the remainder of the cord.
He was transferred to our HARU for ongoing rehabilitation. On admission, he had an indwelling catheter to manage his neurogenic bladder. He received ongoing input by the tissue viability nurses for his heel ulcers. He was hoisted into a regular wheelchair by the ward staff and was on normal diet and fluid. He needed assistance for his washing and dressing. He was able to feed himself for meals.
While at our rehabilitation unit, shoulder pain was reported, described as an ache, worse on mobilisation. This was 60 days since his initial presentation. On examination, the shoulder joint was restricted and painful in all direction. With the possible complication of dysbaric osteonecrosis in mind, an MR shoulder (Fig. 2) was requested to specifically investigate for it. This noted abnormal signal within the humeral shaft, reflecting an infarct, suggesting early osteonecrosis. This was 60 days since his initial presentation. There was also partial thickness rotator cuff tearing with a small full-thickness perforation noted posteriorly at the supraspinatus attachment. X-ray of the entire humerus was performed as per suggestion of the radiology team, which confirmed the diagnosis of early osteonecrosis (Fig. 3).
Fig. 2.
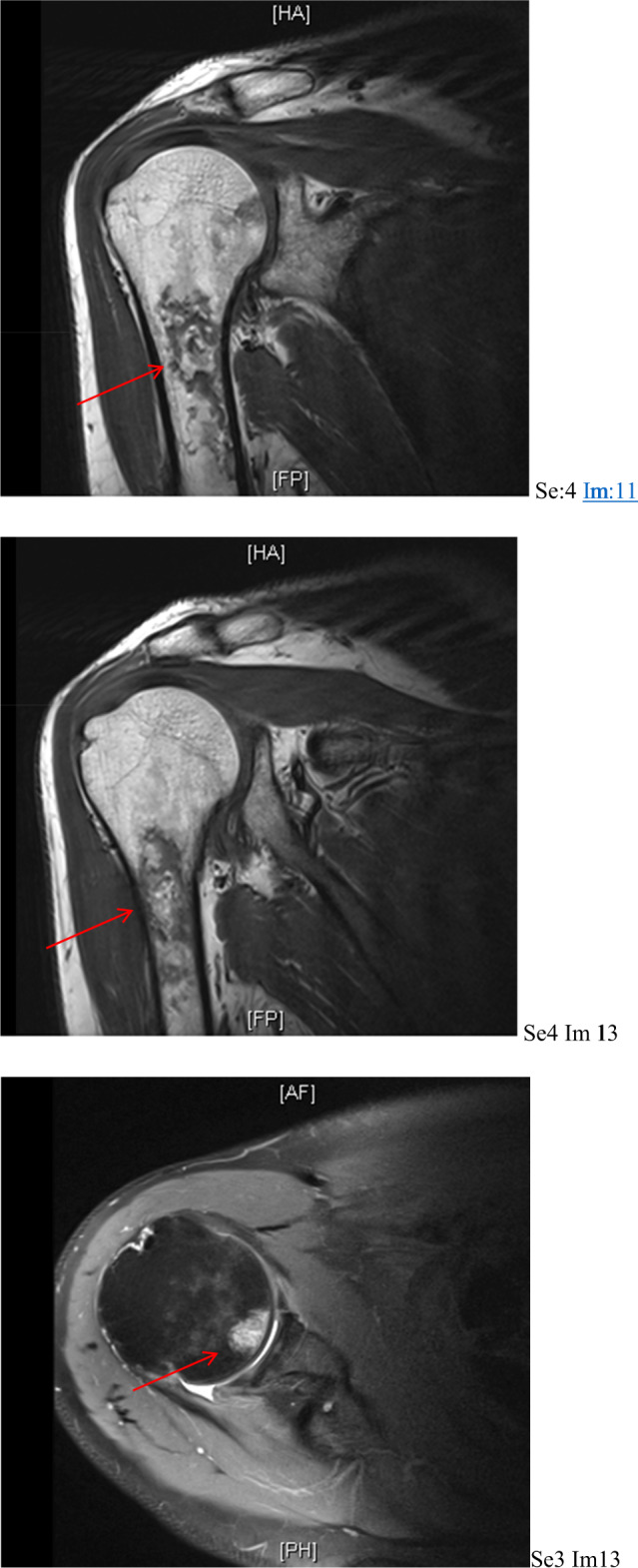
MRI shoulder showing abnormal signal within the humeral shaft, reflecting areas of infarct, in keeping with osteonecrosis.
Fig. 3.
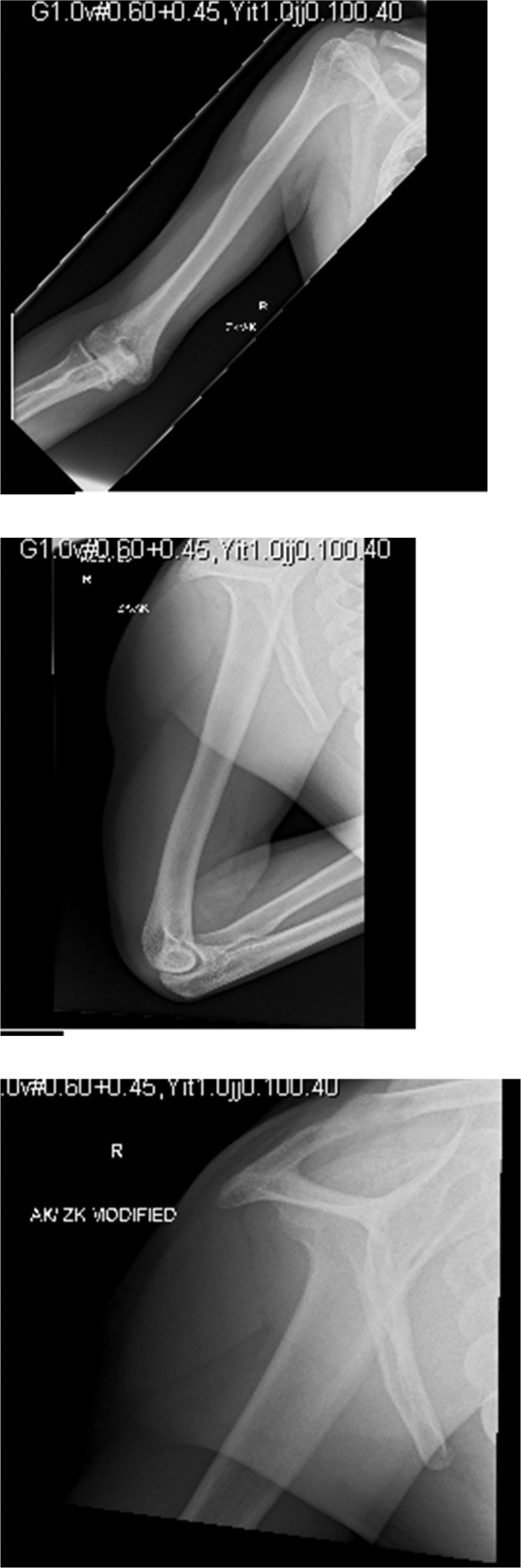
X-ray of humerus confirming the diagnosis of early osteonecrosis.
Discussion
Nitrogen (78%) and oxygen (21%) are the main composition of air. Breathing air that has been compressed under high pressure meant that each breath of the compressed air taken contains more molecules compared to a breath taken at the surface. As oxygen is continuously used by for cell metabolism and anaerobic activities, the extra oxygen molecules do not accumulate in the blood and tissue.
When divers ascend rapidly from a dive or when leaving a compressed air environment, the accumulated nitrogen cannot be exhaled at a speed sufficiently, and this forms bubbles in the blood and tissues. These build-ups of bubbles can subsequently expend as the pressure decreases, injuring tissues, or occluding vessels, leading to pain and various other symptoms. The nitrogen bubbles itself can lead to inflammation, causing swelling and pain in muscles, joints and tendons. Endothelial damage from the gas bubbles further activate the intrinsic clotting cascade, leading to further occlusion [3]. Decompression sickness is not only seen in rapid ascend in divers but also seen in flying after diving, where again there is significant change in air pressures.
There are two types of decompression sickness, type 1 and type 2 [1]. The difference between both is outlined in Table 2. Our patient had type 2 decompression sickness, with spinal cord involvement. He also had brain involvement as in the initial stage, and noted to be bradyphrenic. There was also likely some lung involvement, as he was noted to be dyspnoeic on arrival to the recompression centre.
Table 2.
Difference between decompression sickness.
| Type 1 decompression sickness | Type 2 decompression sickness |
|---|---|
|
• Less severe • Often called the bends • Typically cause pain at joints of the arms or legs, back or muscles • Pain mild or intermittent but may grow stronger and more severe in nature • Less common symptoms ◦ Skin mottling ◦ Swollen lymph nodes ◦ Rash ◦ Extreme fatigue |
• More severe • Commonly result in neurological symptoms such as paralysis and death • Spinal cord extremely vulnerable • Symptoms of spinal cord involvement ◦ Numbness ◦ Tingling ◦ Weakness at arms, legs or both • Symptoms of brain involvement ◦ Headache ◦ Confusion ◦ Trouble speaking ◦ Double vision • Symptoms of inner ear involvement ◦ Vertigo ◦ Tinnitus ◦ Hearing loss • Symptoms of lung involvement ◦ Chest pain ◦ Cough ◦ dyspnoea |
Dysbaric osteonecrosis is a form of non-traumatic avascular necrosis seen in divers and compressed air workers [2, 4]. Multiple theories of aetiologies have been thought to cause dysbaric osteonecrosis, and the most common is thought to be a sub-manifestation of decompressive sickness. Inert gas bubble formation as formerly discussed is thought to cause endothelial disruption and subsequent vascular occlusion through coagulation of intraosseous microcirculation. Venous thrombosis is formed, leading to retrograde arterial flow and increased intraosseous pressure. Avascular necrosis occurs, consequently, leading to chondral microfracture and eventually collapse. An increased hypercoagulable state in certain patient has also been postulated to increase the risk of developing dysbaric osteonecrosis [2, 5]. The shaft of the femur is the most common site to develop dysbaric osteonecrosis, and this is thought to be due to the avascular nature of the femoral head, which increases its risk of developing dysbaric osteonecrosis [6, 7]. The proximal third of the humerus and tibia is also noted to be commonly affected by dysbaric osteonecrosis [8, 9]. There is prospect of revascularisation at the area of osteonecrosis, where granular tissue formed penetrate the necrotic trabeculae, increasing bone strength. Although dysbaric osteonecrosis is often associated with decompression sickness, Walder and colleagues [10] reported only 10% of patients with decompression sickness progressed to dysbaric osteonecrosis. The prevalence of the disease among divers appears to differ between countries, with higher prevalence reported in Turkish and Japanese divers [11]. Regular consumption of alcohol and steroids has been thought to increase the risk for osteonecrosis [12] and is also felt to be a risk factor for dysbaric osteonecrosis [5]. Other risk factors include older age, raised body mass index, increased diving depth and duration, and delay to hyperbaric treatment [13].
X-ray is the initial imaging of choice for diagnosis and evaluation, typically showing decalcification of bone, cystic lesions, osteosclerotic patterns and non-traumatic fractures or bone island [14–16]. MRI is effective at identifying early stages of lesions [17] and is also useful to characterise the nature of the lesion. Changes in imaging’s are seen early as 4 months after hyperbaric exposure and can be seen as late as 8 months to a year [6]. Management depends on the location, severity and specific characteristic of the lesion. No specific staging has been described for dysbaric osteonecrosis involving the humeral head, although the Ficat classification (Table 3A) and Steinberg staging (Table 3B) have been used to stage avascular necrosis of the femoral head [18, 19].
Table 3.
Staging of osteonecrosis.
| Stage | Description |
|---|---|
| (A) Ficat classification | |
| Stage 0 | Normal X-ray, normal MRI, no symptoms |
| Stage 1 | Normal or minor osteopenia on X-ray, oedema on MRI, increased uptake on bone scan, pain in the groin |
| Stage 2 | Mixed osteopenia/sclerosis on X-ray, a defect on MRI, increased uptake on bone scan, groin pain and stiffness on exam |
| Stage 3 | Presents with ‘crescent' sign or some cortical collapse on X-ray, MRI has same findings as X-ray, increased uptake on bone scan, patient has pain radiating to knee and walks with a limp |
| Stage 4 | X-ray shows end-stage collapse with secondary arthrosis of the hip joint, MRI shows similar findings as x-ray, bone scan shows increased uptake, patient presents with pain and a limp. |
| (B) Steinberg staging | |
| Stage 0 | Normal or non-diagnostic radiographs, MRI and bone scan of at risk hip (often contralateral hip involved, or patient has risk factors and hip pain) |
| Stage 1 | Normal radiograph, abnormal bone scan and/or MRI |
| Stage 2 | Cystic and sclerotic radiographic changes |
| Stage 3 | Subchondral lucency or crescent sign |
| Stage 4 |
Flattening of femoral head, with depression graded into • mild: <2 mm • moderate: 2–4 mm • severe: >4 mm |
| Stage 5 | Joint space narrowing with or without acetabular involvement |
| Stage 6 | Advanced degenerative changes |
One should also consider the method of transfer and ambulation of the patient. As there is a risk of pathological fracture, the patient should remain non-weight bearing till reviewed by the orthopaedic team. Analgesics and anti-inflammatory agents tend to be the first line of treatment in mild cases, and in severe cases, surgical treatment such as osteotomy, joint replacement or bone graft can be considered [11]. Patient’s may continue to develop further lesions, despite cessation of exposure to the dysbaric environment and therefore should be monitored [20].
Orthopaedic input was obtained for our patient, and the plan was to monitor his dysbaric necrosis. He was subsequently transferred to our regional spinal rehabilitation unit, practicing transfers with a banana board, with assistance of two during therapy sessions, and being hoisted by the care staff into a standard wheelchair. In view of the high demand of workload on his upper body, his case was re-discussed with the orthopaedic team, and we await the outcome. Kinesio-therapy tape was still used to position the left ankle to maintain range. He was provided with upper limb exercise and support for writing and usage of cutlery, and splints for lower limb were continued, in which the feasibility of the splints due to the heel pressure ulcers were monitored on a regular basis. There was no identifiable speech and language deficit needs or dietetic needs. He received full support for his personal care, medications and positioning needs during his stay till discharge from our unit, which is to be continued by the receiving unit.
We felt that this was an interesting case presentation of dysbaric osteonecrosis as the diagnosis had a significant impact on his rehabilitation prescription. Dysbaric osteonecrosis have been reported as early as 4 weeks post exposure to a hyperbaric environment [8], but also as late as 8 months to a year [6], and indeed been reported to continue to have radiological changes after formal diagnosis [20]. Therefore, there should be an increase in awareness among physicians to consider this diagnosis in patients who have been exposed to a hyperbaric environment.
COMPETING INTERESTS
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Lin Cheng, Email: Lf.cheng@doctors.org.uk.
Pedro Silva, Email: p.silva2@nhs.net.
Raef Dahab, Email: raef.dahab2@srft.nhs.uk.
References
- 1.Moon R. Decompression sickness (Caisson disease; the bends). MSD Manual Professional Version. 2019. https://www.msdmanuals.com/en-gb/professional/injuries-poisoning/injury-during-diving-or-work-in-compressed-air/decompression-sickness. Accessed 24 December 2020.
- 2.White T, Davis D, Cooper J. Dysbaric osteonecrosis. StatPearls; 2020. https://www.ncbi.nlm.nih.gov/books/NBK482310/. Accessed 24 December 2020.
- 3.Cooper J, Hanson K. Decompression sickness. StatPearls Publishing. 2020. https://www.ncbi.nlm.nih.gov/books/NBK537264/. Accessed 24 December 2020. [PubMed]
- 4.Gregg PJ, Walder DN. Caisson disease of bone. Clin Orthop Relat Res. 1986;210:43–54. [PubMed] [Google Scholar]
- 5.Miyanishi K, Kamo Y, Ihra H, et al. Risk factors for dysbaric osteonecrosis. Rheumatology. 2006;4:855–8. doi: 10.1093/rheumatology/kel013. [DOI] [PubMed] [Google Scholar]
- 6.Davidson K. Radiology of dysbaric osteonecrosis. J Clin Pathol. 1972;25:1005–6. doi: 10.1136/jcp.25.11.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davidson JK. Dysbaric osteonecrosis: a reassessment and hypothesis. Med Hypotheses. 2000;54:585–90. doi: 10.1054/mehy.1999.0901. [DOI] [PubMed] [Google Scholar]
- 8.Posadzy M, De Vos N, Vanhoenacker F. Dysbaric osteonecrosis of the humerus. Eurorad. 2017.
- 9.Xue H. Dysbaric osteonecrosis and its radiographic classification in China. Undersea Biomed Res. 1988;15:389–95. [PubMed] [Google Scholar]
- 10.Walder DN. Bone necrosis. In: Jardine FM, McCallum RI, editors. Engineering and health in compressed air work. Proceedings of the international conference. Oxford: E & FN Spon; 1994. p. 16–28.
- 11.Uguen M, Pougnet R et al. Dysbaric osteonecrosis among professional divers: a literature review. UHM V41; 2014. p. 581–9. [PubMed]
- 12.Shah KN, Racine J, et al. Pathophysiology and risk factors for osteonecrosis. Curr Rev Musculoskelet Med. 2015;8:201–9. doi: 10.1007/s12178-015-9277-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gempp E, Louge P, de Maistre S. Predictive factors of dysbaric osteonecrosis following musculoskeletal decompression sickness in recreational SCUBA divers. Jt Bone Spine. 2016;83:357–8. doi: 10.1016/j.jbspin.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 14.Report from the Decompensation Sickness Central Registry and Radiological Panel. Aseptic bone necrosis in commercial divers. Lancet 1981;c318: 348–88. [PubMed]
- 15.Xue HL. Dysbaric osteonecrosis and its radiographic classicifaction in China. Undersea Biomed Res. 1988;15:389–95. [PubMed] [Google Scholar]
- 16.Ota Y, Matsungga H. Bone lesions in divers. J Bone Jt Surg Br. 1974;56:3–16. [PubMed] [Google Scholar]
- 17.Bolte H, Koch A, et al. Detection of dysbaric osteonecrosis in military divers using magnetic resonance imaging. Eur Radiol. 2004;15:368–75. doi: 10.1007/s00330-004-2452-8. [DOI] [PubMed] [Google Scholar]
- 18.Rozmiarek J, Rezaee A, et al. Steinberg staging of avascular necrosis. Radiopenia. https://radiopaedia.org/articles/steinberg-staging-of-avascular-necrosis-1?lang=gb. Accessed 24 December 2020.
- 19.El-Feky M, Gaillard F. Ficat and Arlet classification of avascular necrosis of femoral head. Radiopenia. https://radiopaedia.org/articles/ficat-and-arlet-classification-of-avascular-necrosis-of-femoral-head?lang=gb. Accessed 24 December 2020.
- 20.Van Blarcom ST, Czarnecki DJ, et al. Does dysbaric osteonecrosis progress in the absence of further hyperbaric exposure? A 10-year radiological follow up of 15 patients. AJR Am J Roentgenol. 1990;155:95–7. doi: 10.2214/ajr.155.1.2112875. [DOI] [PubMed] [Google Scholar]



