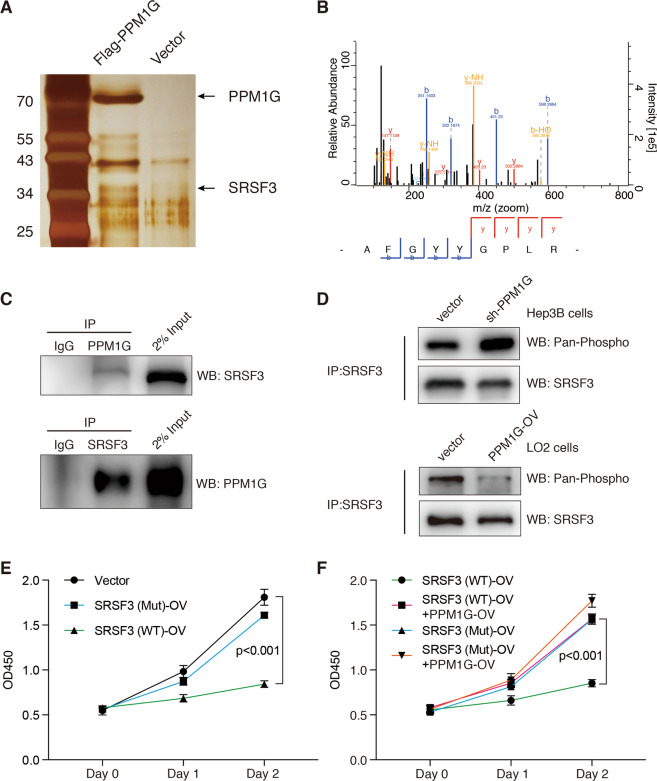
Fig. 5. PPM1G interacts with SRSF3 in Hep3B cells.
A–B Identify PPM1G-interacted proteins by immunoprecipitation of Flag-tagged PPM1G protein followed with LC-MS/MS. The silver staining of Flag-PPM1G immunoprecipitated proteins was shown (A). Representative peptides of the SRSF3 protein were illustrated (B). C Co-immunoprecipitation showed direct interaction between PPM1G and SRSF3. Antibodies against PPM1G or SRSF3 were used for immunoprecipitation. IgG was used as the negative control. About 2% of input proteins were loaded as an internal control. D PPM1G dephosphorylated the SRSF3. Hep3B cells were transfected with shRNA-targeting PPM1G or vector (upper panel). LO2 cells were transfected with PPM1G overexpression plasmid or vector (lower panel). All SRSF3 proteins were first immunoprecipitated using antibodies against SRSF3. The phosphorylated levels of SRSF3 were then detected using antibodies against pan-phosphorylated proteins. E Overexpression of SRSF3-WT inhibited Hep3B cell growth. Hep3B cells were transfected with SRSF3-WT, SRSF3-Mut, or vector plasmid. F PPM1G overexpression abolished SRSF3-WT-induced cell growth inhibition. Hep3B cells were co-transfected with SRSF3-WT/SRSF3-Mut and PPM1G overexpression plasmid. Viable cells were examined at 0-, 1-, and 2-days post transfection by CCK-8 assays.